Reparixin as a Potential Antiepileptogenic Agent: Modulation of the CXCL1-CXCR1/2 Axis and Seizure Activity in a Kindling Rat Model of Temporal Lobe Epilepsy
- PMID: 40243443
- PMCID: PMC11989020
- DOI: 10.3390/ijms26072831
Reparixin as a Potential Antiepileptogenic Agent: Modulation of the CXCL1-CXCR1/2 Axis and Seizure Activity in a Kindling Rat Model of Temporal Lobe Epilepsy
Abstract
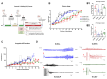
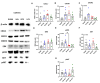
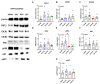
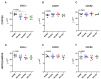
Chemokine (CXC motif) ligand 8 (CXCL8) is a pro-inflammatory chemokine binding to CXC motif receptors 1/2 (CXCR1/2). Patients with temporal lobe epilepsy (TLE) exhibit increased serum CXCL8 levels. CXC motif ligand 1 (CXCL1), a murine ortholog of CXCL8, has been implicated in seizure generation and neuronal loss. This study evaluated the antiepileptogenic and antiseizure effects of reparixin in amygdaloid kindling rat model of TLE. Reparixin was administered during the kindling period for 14 days, and seizures were induced twice daily via electrical stimulation. To assess the antiseizure effects, reparixin was administered to fully kindled animals, and stimulations were performed 24 and 48 h later. Levetiracetam, a broad-spectrum antiseizure drug, was administered intraperitoneally (i.p.) as positive control 1 h before each stimulation. Reparixin delayed secondary seizure generalization during kindling. Reparixin reduced seizure severity and after-discharge duration in fully kindled animals at 24 h from treatment initiation. CXCR1/2 and protein kinase B pathway proteins exhibited no significant changes; reparixin reduced the phospho-extracellular signal-regulated kinase (pERK)/ERK ratio in the cortex and hippocampus. CXCL1 expression was significantly decreased in the cortex. Reparixin exhibited antiepileptogenic and partial antiseizure effects by modulating the CXCL1-CXCR1/2 axis and reducing ERK signaling. Already in clinical trials on respiratory diseases, reparixin could be repurposed for epilepsy therapy.
Keywords: CXCL1; CXCR1/2 antagonist; amygdaloid kindling; antiepileptogenic; antiseizure therapy; neuroinflammation; reparixin.
Conflict of interest statement
L. Brandolini, A. Aramini, M. Allegretti and L. De Filippis are employed by Dompe’ Farmaceutici S.p.a. The other authors declare no conflicts of interest.
Figures

References
-
- Motovilov K., Maguire C., Briggs D., Melamed E. Altered cytokine profile in clinically suspected seronegative autoimmune associated epilepsy. medRxiv. 2024 doi: 10.1101/2024.09.13.24310337. - DOI
-
- Jauhari P., Kaur P., Gulati S., Meena A.K., Pandey T., Upadhyay A. Diagnostic and prognostic significance of serum interleukins in epileptic encephalopathy with spike wave activation in sleep (EE-SWAS) syndrome. Eur. J. Paediatr. Neurol. 2024;53:33–38. doi: 10.1016/j.ejpn.2024.09.006. - DOI - PubMed
-
- Milano C., Montali M., Barachini S., Burzi I.S., Pratesi F., Petrozzi L., Chico L., Morganti R., Gambino G., Rossi L., et al. Increased production of inflammatory cytokines by circulating monocytes in mesial temporal lobe epilepsy: A possible role in drug resistance. J. Neuroimmunol. 2024;386:578272. doi: 10.1016/j.jneuroim.2023.578272. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

