Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases
- PMID: 40243512
- PMCID: PMC11988947
- DOI: 10.3390/ijms26072916
Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases
Abstract
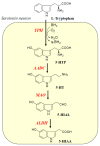
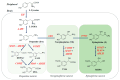
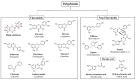
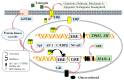
Serotonin (5-HT), dopamine (DA), and norepinephrine (NE) are key monoamine neurotransmitters regulating behaviors, mood, and cognition. 5-HT affects early brain development, and its dysfunction induces brain vulnerability to stress, raising the risk of depression, anxiety, and autism in adulthood. These neurotransmitters are synthesized from tryptophan and tyrosine via hydroxylation and decarboxylation, and are metabolized by monoamine oxidase (MAO). This review aims to summarize the current findings on the role of dietary phytochemicals in modulating monoamine neurotransmitter biosynthesis, metabolism, and function, with an emphasis on their potential therapeutic applications in neuropsychiatric disorders. Phytochemicals exert antioxidant, neurotrophic, and neurohormonal activities, regulate gene expression, and induce epigenetic modifications. Phytoestrogens activate the estrogen receptors or estrogen-responsive elements of the promoter of target genes, enhance transcription of tryptophan hydroxylase and tyrosine hydroxylase, while inhibiting that of MAO. These compounds also influence the interaction between genetic and environmental factors, potentially reversing dysregulated neurotransmission and the brain architecture associated with neuropsychiatric conditions. Despite promising preclinical findings, clinical applications of phytochemicals remain challenging. Advances in nanotechnology and targeted delivery systems offer potential solutions to enhance clinical efficacy. This review discusses mechanisms, challenges, and strategies, underscoring the need for further research to advance phytochemical-based interventions for neuropsychiatric diseases.
Keywords: catecholamines; monoamine oxidase; neuropsychiatric diseases; neurotransmission; phytochemicals; serotonin; tryptophan hydroxylase; tyrosine hydroxylase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Monoamine neurocircuitry in depression and strategies for new treatments.Prog Neuropsychopharmacol Biol Psychiatry. 2013 Aug 1;45:54-63. doi: 10.1016/j.pnpbp.2013.04.009. Epub 2013 Apr 19. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 23602950 Review.
-
Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: from neurotransmitter imbalance to impaired neurogenesis.J Neural Transm (Vienna). 2018 Jan;125(1):53-66. doi: 10.1007/s00702-017-1709-8. Epub 2017 Mar 14. J Neural Transm (Vienna). 2018. PMID: 28293733 Review.
-
Amitraz changes NE, DA and 5-HT biosynthesis and metabolism mediated by alterations in estradiol content in CNS of male rats.Chemosphere. 2017 Aug;181:518-529. doi: 10.1016/j.chemosphere.2017.04.113. Epub 2017 Apr 26. Chemosphere. 2017. PMID: 28463726
-
Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: genetic and environmental factors involved in type A MAO expression.J Neural Transm (Vienna). 2016 Feb;123(2):91-106. doi: 10.1007/s00702-014-1362-4. Epub 2015 Jan 22. J Neural Transm (Vienna). 2016. PMID: 25604428 Review.
-
[Effect of Rehmanniae Radix on depression-like behavior and hippocampal monoamine neurotransmitters of chronic unpredictable mild stress model rats].Zhongguo Zhong Yao Za Zhi. 2022 Sep;47(17):4691-4697. doi: 10.19540/j.cnki.cjcmm.20220421.401. Zhongguo Zhong Yao Za Zhi. 2022. PMID: 36164876 Chinese.
Cited by
-
Exploring the therapeutic role of Moringa oleifera in neurodegeneration: antioxidant, anti-inflammatory, and neuroprotective mechanisms.Inflammopharmacology. 2025 Jul;33(7):3653-3669. doi: 10.1007/s10787-025-01794-y. Epub 2025 May 31. Inflammopharmacology. 2025. PMID: 40448817 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

