The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence
- PMID: 40243524
- PMCID: PMC11988320
- DOI: 10.3390/ijms26072883
The Role of FpfetC from Fusarium proliferatum in Iron Acquisition, Fumonisin B1 Production, and Virulence
Abstract
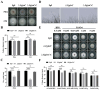
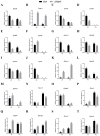
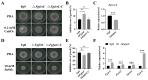
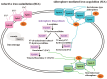
Iron is an essential micronutrient required for the fungal growth and propagation. Fusarium proliferatum is the causal agent of rice spikelet rot disease. In this study, we characterized the role of F. proliferatum multicopper ferroxidase (FpfetC), which mediated the oxidization of ferrous to ferric iron in the reductive system of iron assimilation. Deletion of FpfetC led to impaired growth under iron-deprived conditions, and the growth defect could be restored by exogenous iron. Compared to wild-type Fp9 strain, ΔFpfetC showed increased conidiation, resistance to copper stress, and sensitivity to zinc stress. FpfetC deficiency rendered a transcription remodeling of genes involved in high-affinity iron assimilation, iron homeostasis and iron storage. Moreover, production of fumonisin B1 (FB1) and transcript levels of fumonisin biosynthesis (Fpfums) genes were elevated in ΔFpfetC. ΔFpfetC exhibited hypervirulence to rice, accompanied with aggravation of invasive hyphae and activation of siderophore synthesis at the sites of inoculation. Additionally, disruption of FpfetC attenuated penetration ability to cellophane membrane under iron starvation. Taken together, these results demonstrated that FpfetC played important roles in iron uptake, conidiation, response to metal stress, fumonisin biosynthesis, and virulence in F. proliferatum.
Keywords: Fusarium proliferatum; fumonisins; iron acquisition; multicopper ferroxidase; virulence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
New insights into fumonisin production and virulence of Fusarium proliferatum underlying different carbon sources.Food Res Int. 2019 Feb;116:397-407. doi: 10.1016/j.foodres.2018.08.053. Epub 2018 Aug 24. Food Res Int. 2019. PMID: 30716962
-
Effects of Disruption of Five FUM Genes on Fumonisin Biosynthesis and Pathogenicity in Fusarium proliferatum.Toxins (Basel). 2019 Jun 7;11(6):327. doi: 10.3390/toxins11060327. Toxins (Basel). 2019. PMID: 31181598 Free PMC article.
-
Glycosyltransferase FvCpsA Regulates Fumonisin Biosynthesis and Virulence in Fusarium verticillioides.Toxins (Basel). 2021 Oct 11;13(10):718. doi: 10.3390/toxins13100718. Toxins (Basel). 2021. PMID: 34679011 Free PMC article.
-
Experimental infection of Fusarium proliferatum in Oryza sativa plants; fumonisin B1 production and survival rate in grains.Int J Food Microbiol. 2012 Jun 1;156(3):204-8. doi: 10.1016/j.ijfoodmicro.2012.03.021. Epub 2012 Mar 28. Int J Food Microbiol. 2012. PMID: 22534354
-
FvSO regulates vegetative hyphal fusion, asexual growth, fumonisin B1 production, and virulence in Fusarium verticillioides.Fungal Biol. 2015 Dec;119(12):1158-1169. doi: 10.1016/j.funbio.2015.08.013. Epub 2015 Sep 3. Fungal Biol. 2015. PMID: 26615739
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

