Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus
- PMID: 40243525
- PMCID: PMC11988775
- DOI: 10.3390/ijms26072912
Glycyl-tRNA Synthetase as a Target for Antiviral Drug Screening Against Influenza Virus
Abstract
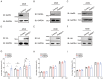
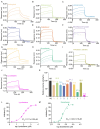
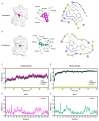
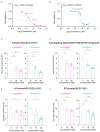
Influenza viruses are characterized by their high variability and pathogenicity, and effective therapeutic options remain limited. Given these challenges, targeting host cell proteins that facilitate viral replication presents a promising strategy for antiviral drug discovery. In the present study, we observed a significant upregulation of Glycyl-tRNA synthetase (GlyRS) within 24 h post-PR8 virus infection. The inhibition of GlyRS expression in A549 cells resulted in a marked reduction in infection rates across multiple influenza virus strains, while the overexpression of GlyRS led to an increase in viral infectivity during the early stages of infection. These findings suggest that GlyRS plays a critical role in the replication of influenza virus. Accordingly, we screened for potential inhibitors targeting GlyRS and identified Lycobetaine and Scutellarein using a multifaceted approach. Through a combination of molecular dynamics simulations, we further elucidated the mechanisms of action and potential binding sites of these compounds. Both inhibitors effectively suppressed the replication of influenza viruses, and their antiviral activity was confirmed to be mediated by GlyRS targeting. Therefore, GlyRS inhibitors, such as Lycobetaine and Scutellarein, represent promising candidates for combating influenza infections and provide novel insights into the treatment of influenza and aaRS-related diseases, opening new avenues for the development of aaRS-targeted therapeutics.
Keywords: Glycyl-tRNA synthetase; antiviral drug screening; influenza virus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
New-generation screening assays for the detection of anti-influenza compounds targeting viral and host functions.Antiviral Res. 2013 Oct;100(1):120-32. doi: 10.1016/j.antiviral.2013.07.018. Epub 2013 Aug 6. Antiviral Res. 2013. PMID: 23933115 Free PMC article. Review.
-
Screening and identification of inhibitors against influenza A virus from a US drug collection of 1280 drugs.Antiviral Res. 2014 Sep;109:54-63. doi: 10.1016/j.antiviral.2014.06.007. Epub 2014 Jun 24. Antiviral Res. 2014. PMID: 24971493
-
Discovery of a Novel Specific Inhibitor Targeting Influenza A Virus Nucleoprotein with Pleiotropic Inhibitory Effects on Various Steps of the Viral Life Cycle.J Virol. 2021 Apr 12;95(9):e01432-20. doi: 10.1128/JVI.01432-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33627391 Free PMC article.
-
Antiviral efficacy of nanoparticulate vacuolar ATPase inhibitors against influenza virus infection.Int J Nanomedicine. 2018 Dec 14;13:8579-8593. doi: 10.2147/IJN.S185806. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30587980 Free PMC article.
-
Type II transmembrane serine proteases as potential target for anti-influenza drug discovery.Expert Opin Drug Discov. 2017 Nov;12(11):1139-1152. doi: 10.1080/17460441.2017.1372417. Epub 2017 Sep 5. Expert Opin Drug Discov. 2017. PMID: 28870104 Review.
References
-
- Bonomini A., Felicetti T., Pacetti M., Bertagnin C., Coletti A., Giammarino F., De Angelis M., Poggialini F., Macchiarulo A., Sabatini S., et al. Optimization of Potent, Broad-spectrum, and Specific Anti-influenza Compounds Targeting RNA Polymerase PA-PB1 heterodimerization. Eur. J. Med. Chem. 2024;277:116737. doi: 10.1016/j.ejmech.2024.116737. - DOI - PubMed
-
- Teixeira Alves L.G., Baumgardt M., Langner C., Fischer M., Maria Adler J., Bushe J., Firsching T.C., Mastrobuoni G., Grobe J., Hoenzke K., et al. Protective role of the HSP90 inhibitor, STA-9090, in lungs of SARS-CoV-2-infected Syrian golden hamsters. BMJ Open Respir. Res. 2024;11:e001762. doi: 10.1136/bmjresp-2023-001762. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

