Decoding PHR-Orchestrated Stress Adaptation: A Genome-Wide Integrative Analysis of Transcriptional Regulation Under Abiotic Stress in Eucalyptus grandis
- PMID: 40243569
- PMCID: PMC11988722
- DOI: 10.3390/ijms26072958
Decoding PHR-Orchestrated Stress Adaptation: A Genome-Wide Integrative Analysis of Transcriptional Regulation Under Abiotic Stress in Eucalyptus grandis
Abstract
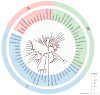
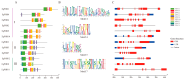
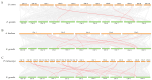
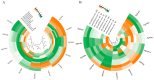
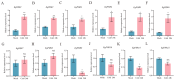
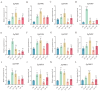
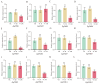
The phosphate starvation response (PHR) transcription factor family play central regulatory roles in nutrient signaling, but its relationship with other abiotic stress remains elusive. In the woody plant Eucalyptus grandis, we characterized 12 EgPHRs, which were phylogenetically divided into three groups, with group I exhibiting conserved structural features (e.g., unique motif composition and exon number). Notably, a protein-protein interaction network analysis revealed that EgPHR had a species-specific protein-protein interaction network: EgPHR6 interacted with SPX proteins of multiple species, while Eucalyptus and poplar PHR uniquely bound to TRARAC-kinesin ATPase, suggesting functional differences between woody and herbaceous plants. A promoter sequence analysis revealed a regulatory network of 59 transcription factors (TFs, e.g., BPC, MYBs, ERFs and WUS), mainly associated with tissue differentiation, abiotic stress, and hormonal responses that regulated EgPHRs' expression. Transcriptomics and RT-qPCR gene expression analyses showed that all EgPHRs dynamically responded to phosphate (Pi) starvation, with the expression of EgPHR2 and EgPHR6 exhibiting sustained induction, and were also regulated by salt, cold, jasmonic acid, and boron deficiency. Strikingly, nitrogen starvation suppressed most EgPHRs, highlighting crosstalk between nutrient signaling pathways. These findings revealed the multifaceted regulatory role of EgPHRs in adaptation to abiotic stresses and provided insights into their unique evolutionary and functional characteristics in woody plants.
Keywords: Eucalyptus grandis; PHR; boron deficiency; cold stress; gene expression; nitrogen starvation; phosphate starvation; salt stress; transcription factor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Meharg A.A. In: Marschner’s Mineral Nutrition of Higher Plants. 3rd ed. Marschner P., editor. Elsevier; Amsterdam, The Netherlands: Academic Press; Amsterdam, The Netherlands: 2011. p. 684.
MeSH terms
Substances
Grants and funding
- CAFYBB2022SY017/the Fundamental Research Funds for the Central Non-profit Research Institution of CAF
- 2022YFD2200203/the National Key Research and Development Program of China during the 14th Five-year Plan Period
- 72202200205/the Forestry Peak Discipline Construction Project of Fujian Agriculture and Forestry University
- 2022SF-587/the Key Research and Development Program of Shaanxi
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

