Experimental Conditions to Retrieve Intrinsic Cooperativity α Directly from Single Binding Assay Data Exemplified by the Ternary Complex Formation of FKBP12, MAPRE1 and Macrocyclic Molecular Glues
- PMID: 40243574
- PMCID: PMC11989212
- DOI: 10.3390/ijms26072936
Experimental Conditions to Retrieve Intrinsic Cooperativity α Directly from Single Binding Assay Data Exemplified by the Ternary Complex Formation of FKBP12, MAPRE1 and Macrocyclic Molecular Glues
Abstract
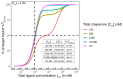
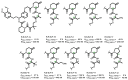
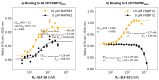
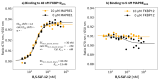
The incorporation of disease-relevant targets into ternary complexes in a compound-dependent manner by utilizing an assisting chaperone has become a common modality as far as bifunctional ternary complex-forming compounds are concerned. In contrast, examples of ternary complexes formed by molecular glues are much rarer. Due to their lack of significant binary (independent) target affinity, their identification cannot yet be achieved by rational methods and is, therefore, much more challenging. However, it is precisely for that reason (given the associated advantages) that their systematic identification and application in drug discovery has recently attracted particular interest. In contrast to bifunctional ternary complex-forming compounds, molecular glues retrieve a significant part of their thermodynamic stability through newly induced chaperone-target or glue-target interactions that occur only in the ternary complex. These interactions lead to enhanced ligand binding-termed intrinsic cooperativity α-which can be retrieved via the apparent cooperativity either by monitoring ligand binding through the chaperone or through the target protein. In this publication, the advantage of measuring the apparent cooperativity (to determine the cooperativity α) by the weaker binding protein is discussed and illustrated using the example of ternary complexes between FKBP12, MAPRE1 and macrocyclic molecular glues derived from the rapamycin binding motif for FKBP12. Furthermore, the impact of the following three parameters on the apparent cooperativity is illustrated: (1) the concentration of the monitoring protein, (2) the excess of the counter protein, and (3) the affinity of the glue to the weaker binding protein in combination with the degree of intrinsic cooperativity α. From this, experimental conditions to determine the intrinsic cooperativity α with only one binding assay and without the need for a comprehensive mathematical model covering all simultaneous events under non-saturating conditions are highlighted. However, this framework requires a binding assay capable of measuring or at least estimating very weak binary affinities. If this is not possible for experimental reasons, but binding assays for both proteins are available within a normal bandwidth and the affinity to the stronger binding protein is not too high, it is discussed how the binding curve for the weaker binding protein in the presence of an excess of the weaker binding protein can be used to overcome the missing binary Kd for the weakly binding protein.
Keywords: FKBP12; MAPRE1; cooperativity; measuring weak affinities; spectral shift method; ternary complexes.
Conflict of interest statement
Hans-Jörg Roth, Richard Stein, Marianne Fouché, Julian Wong and Michael Salcius were employed by Novartis AG, Switzerland. Jan Schnatwinkel was employed by NanoTemper Technologies GmbH, Germany. Chen Shu-Yu was employed by ETH Zürich, Switzerland. We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures













References
-
- Li F., Aljahdali I.A.M., Ling X. Molecular Glues: Capable Protein-Binding Small Molecules That Can Change Protein-Protein Interactions and Interactomes for the Potential Treatment of Human Cancer and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022;23:6206. doi: 10.3390/ijms23116206. - DOI - PMC - PubMed
-
- Weagel E.G., Foulks J.M., Siddiqui A., Warner S.L. Molecular glues: Enhanced protein-protein interactions and cell proteome editing. Med. Chem. Res. 2022;31:1068–1087. doi: 10.1007/s00044-022-02882-2. - DOI
-
- Geiger T.M., Schäfer S.C., Dreizler J.K., Walz M., Hausch F. Clues to molecular glues. Curr. Res. Chem. Biol. 2022;2:100018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

