Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis
- PMID: 40243608
- PMCID: PMC11988463
- DOI: 10.3390/ijms26072964
Synergistic Effects of Oxaliplatin, 5-Fluorouracil, and Novel Synthetic Uracil Analog U-359 on Breast Cancer Cell Carcinogenesis
Abstract
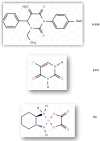
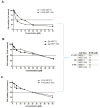
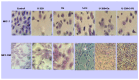
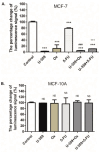
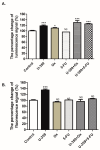
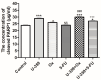
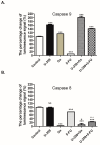
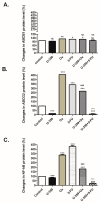
Breast cancer presents significant global challenges, necessitating effective treatments to combat drug resistance and minimize chemotherapy side effects. This study evaluated the cytotoxic effects of U-359, Oxaliplatin (Ox), and 5-Fluorouracil (5-FU) in MCF-7 and MCF-10A cells using MTT and RealTime-GLO assays. Morphological changes were assessed by light microscopy following Wright-Giemsa staining. Apoptosis induction was studied using qPCR for apoptotic markers, the RealTime-Glo™ Annexin V assay, and the cleaved PARP1 ELISA assay. Caspase 8 and 9 activities, ABCB1, ABCG2, and NF-κB protein levels were quantified using ELISA. Synergy was analyzed using the Bliss Independence Model. The results indicated that combining U-359 with Ox and 5-FU enhanced cytotoxicity compared to individual treatments. U-359 induced apoptosis-associated morphological changes in MCF-7 cells, which were augmented with the Ox and 5-FU treatment. Apoptosis assays confirmed the up-regulation of pro-apoptotic markers and the down-regulation of anti-apoptotic markers with U-359 alone or in combination. Elevated cleaved PARP1 levels suggested robust apoptosis induction with U-359 and Ox or 5-FU. Caspase activity assays demonstrated a significant activation of caspase 8 and 9, implicating both apoptotic pathways. Furthermore, U-359 down-regulated ABCB1, ABCG2, and NF-κB in MCF-7 cells, which were up-regulated by Ox and 5-FU alone. The Bliss Independence Model revealed strong synergistic interactions (SI < 1) between U-359 and Ox or 5-FU, particularly in reducing ABCB1 and NF-κB levels. U-359 combined with Ox and 5-FU shows potential for overcoming chemotherapy resistance in breast cancer by enhancing apoptosis and modulating drug resistance. Further clinical studies are needed to optimize treatment and improve outcomes.
Keywords: 5-fluorouracil; MCF-7; U-359; anticancer compounds; apoptosis; multidrug resistance; oxalipaltin.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil.Cell Death Dis. 2013 Feb 21;4(2):e505. doi: 10.1038/cddis.2013.26. Cell Death Dis. 2013. PMID: 23429291 Free PMC article.
-
Combination of 5-fluorouracil and Lipopolysaccharide Synergistically Induces Cytotoxicity and Apoptosis in MCF-7 Human Breast Cancer Cells.Iran J Allergy Asthma Immunol. 2020 Aug 25;19(4):426-436. doi: 10.18502/ijaai.v19i4.4117. Iran J Allergy Asthma Immunol. 2020. PMID: 33463109
-
5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment.Mol Cell Biochem. 2018 Feb;439(1-2):189-198. doi: 10.1007/s11010-017-3147-1. Epub 2017 Aug 9. Mol Cell Biochem. 2018. PMID: 28795251
-
Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition.Biomed Pharmacother. 2019 Jun;114:108855. doi: 10.1016/j.biopha.2019.108855. Epub 2019 Apr 16. Biomed Pharmacother. 2019. PMID: 31003140
-
Survivin up-regulates the expression of breast cancer resistance protein (BCRP) through attenuating the suppression of p53 on NF-κB expression in MCF-7/5-FU cells.Int J Biochem Cell Biol. 2013 Sep;45(9):2036-44. doi: 10.1016/j.biocel.2013.06.026. Epub 2013 Jul 6. Int J Biochem Cell Biol. 2013. PMID: 23838170
References
-
- Pectasides D., Pectasides M., Farmakis D., Bountouroglou N., Nikolaou M., Koumpou M., Mylonakis N., Kosmas C. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil in pretreated advanced breast cancer: A phase II study. Ann. Oncol. 2003;14:537–542. - PubMed
-
- Oshaughnessy J.A., Blum J., Moiseyenko V., Jones S.E., Miles D., Bell D., Rosso R., Mauriac L., Osterwalder B., Burger H.U., et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann. Oncol. 2001;12:1247–1254. doi: 10.1023/A:1012281104865. - DOI - PubMed
-
- Bajetta E., Procopio G., Celio L., Gattinoni L., Della Torre S., Mariani L., Catena L., Ricotta R., Longarini R., Zilembo N., et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J. Clin. Oncol. 2005;23:2155–2161. doi: 10.1200/JCO.2005.02.167. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

