Administration of Noggin Suppresses Fibrinogen Leakage into the Brain in the Acute Phase After Traumatic Brain Injury in Mice
- PMID: 40243640
- PMCID: PMC11988522
- DOI: 10.3390/ijms26073002
Administration of Noggin Suppresses Fibrinogen Leakage into the Brain in the Acute Phase After Traumatic Brain Injury in Mice
Abstract
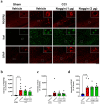
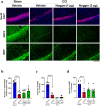
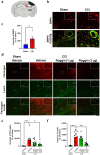
Traumatic brain injury (TBI) causes neurovascular unit (NVU) dysfunction, including hyperpermeability of the blood-brain barrier to fibrinogen, glial activation, and neuronal damage, possibly leading to secondary brain damage. However, no known substance can inhibit its pathogenesis. In this study, we investigated noggin, a bone morphogenetic protein (BMP) 4 inhibitor, as a TBI pathogenesis-inhibiting substance. We induced acute TBI in C57BL/6J mice through a controlled cortical impact (CCI) and evaluated the effects of noggin on fibrinogen leakage into the brain and NVU-constituting cells, including pericytes, microglia, astrocytes, and neurons. CCI mice showed increased BMP4 levels and extravascular fibrinogen in the hippocampus. Noggin treatment significantly suppressed fibrinogen leakage four days post-CCI in a dose-dependent manner. Immunofluorescence staining revealed that noggin administration did not inhibit the activation of NVU cells such as pericytes, microglia, and astrocytes, which were characterized by increased PDGFRβ, Iba1, and GFAP expression levels, respectively. On postoperative day 4, CCI mice showed neuronal cell and myelinated neuronal fiber loss, which were not significantly affected by noggin administration. In conclusion, noggin administration suppresses fibrinogen leakage into the brain in the acute phase after TBI. However, the suppression of fibrinogen leakage through noggin administration did not alleviate neuronal damage and activation of NVU cells during the acute phase of TBI.
Keywords: bone morphogenetic protein 4; fibrinogen leakage; neuronal damage; neurovascular unit; noggin; traumatic brain injury.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Pearn M.L., Niesman I.R., Egawa J., Sawada A., Almenar-Queralt A., Shah S.B., Duckworth J.L., Head B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2017;37:571–585. doi: 10.1007/s10571-016-0400-1. - DOI - PMC - PubMed
-
- Jochems D., van Rein E., Niemeijer M., van Heijl M., van Es M.A., Nijboer T., Leenen L.P.H., Houwert R.M., van Wessem K.J.P. Incidence, causes and consequences of moderate and severe traumatic brain injury as determined by Abbreviated Injury Score in the Netherlands. Sci. Rep. 2021;11:19985. doi: 10.1038/s41598-021-99484-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

