Control of Conformational Transitions by the Conserved GX9P Motif in the Fifth Transmembrane Domain of Neurotransmitter Sodium Symporters
- PMID: 40243663
- PMCID: PMC11988846
- DOI: 10.3390/ijms26073054
Control of Conformational Transitions by the Conserved GX9P Motif in the Fifth Transmembrane Domain of Neurotransmitter Sodium Symporters
Abstract
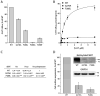
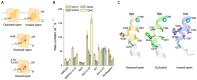
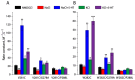
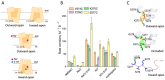
The neurotransmitter sodium symporters (NSSs) play critical roles in the neurotransmission of monoamine and amino acid neurotransmitters and are the molecular targets of therapeutic agents in the treatment of several psychiatric disorders. Despite significant progress in characterizing structures and transport mechanisms, the management of conformational transitions by structural elements coupled with ion and substrate binding remains to be fully understood. In the present study, we biochemically identified a conserved GX9P motif in the fifth transmembrane domain (TM5) of the serotonin transporter (SERT) that plays a vital role in its transport function by facilitating conformational transitions. Mutations of the conserved Gly278 or Pro288 in the GX9P motif dramatically decreased specific transport activity by reducing the substrate binding-induced conformational transitions from an outward-open to an inward-open conformation. In addition, cysteine accessibility measurements demonstrated that the unwinding of the intracellular part of TM5 occurs during conformational transitions from an outward-open state, through an occluded state, to an inward-open state and that substrate binding triggers TM5 unwinding. Furthermore, mutations of the GX9P motif were shown to result in destructive effects on TM5 unwinding, suggesting that the GX9P motif controls conformational transitions through TM5 unwinding. Taken together, the present study provides new insights into the structural elements controlling conformational transitions in NSS transporters.
Keywords: GX9P motif; TM5 unwinding; conformational transition; neurotransmitter sodium symporter; serotonin transporter; transport mechanism.
Conflict of interest statement
The authors declare no conflicts of interest with the contents of this article.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

