Rhusflavone Modulates Osteoclastogenesis Through RANKL-Induced AKT Signaling in Bone Marrow-Derived Macrophages
- PMID: 40243668
- PMCID: PMC11988637
- DOI: 10.3390/ijms26073025
Rhusflavone Modulates Osteoclastogenesis Through RANKL-Induced AKT Signaling in Bone Marrow-Derived Macrophages
Erratum in
-
Correction: Yun et al. Rhusflavone Modulates Osteoclastogenesis Through RANKL-Induced AKT Signaling in Bone Marrow-Derived Macrophages. Int. J. Mol. Sci. 2025, 26, 3025.Int J Mol Sci. 2025 Jul 30;26(15):7354. doi: 10.3390/ijms26157354. Int J Mol Sci. 2025. PMID: 40806792 Free PMC article.
Abstract
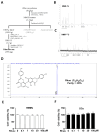
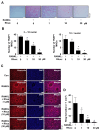
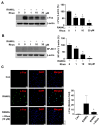
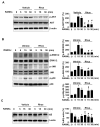
Osteoclast differentiation inhibition is a viable treatment strategy for osteoporosis because osteoclasts play a vital role in disease progression. Rhusflavone (Rhus), a biflavonoid, exhibits a sedative-hypnotic effect via the positive allosteric modulation of GABA(A) receptors. Although several biflavonoids possess activities that help prevent bone loss, the potential effects of Rhus on osteoclastogenesis have not been reported yet. In this study, we investigated the effects and underlying biological mechanisms of Rhus isolated from the dried roots of Rhus succedanea on osteoclastogenesis in primary cultured bone marrow-derived macrophages. No cytotoxicity was observed in bone marrow macrophages (BMMs) or during osteoclast differentiation. However, Rhus reduced the number of tartrate-resistant acid phosphatase (TRAP)-positive multinuclear osteoclasts during receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclastogenesis. The results of F-actin ring formation demonstrated that Rhus suppresses the bone resorption activity of osteoclasts. Additionally, Rhus inhibits the expression of osteoclast differentiation marker proteins, specifically c-Fos and NF-ATc1. Western blot analysis revealed that Rhus primarily attenuated RANKL-mediated key signaling pathways, particularly the AKT signaling pathway. Furthermore, we found that the AKT activator and inhibitor pharmacologically abolished and enhanced the inhibitory effects of Rhus on osteoclast differentiation, respectively. Taken together, our findings provide evidence that Rhus is a promising biologically active compound that regulates osteoclast differentiation by inhibiting the AKT signaling pathway, which may contribute to future drug development.
Keywords: AKT; BMM; RANKL; Rhusflavone; bone; osteoclast.
Conflict of interest statement
Hyung-Mun Yun and Kyung-Ran Park are quest editors for IJMS, special issue “Molecular Research on Apoptosis and Autophagy in Osteosarcoma”. The guest editors declare that there are no conflicts of interest related to the editorial process of this research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Downregulation of the metalloproteinases ADAM10 or ADAM17 promotes osteoclast differentiation.Cell Commun Signal. 2024 Jun 11;22(1):322. doi: 10.1186/s12964-024-01690-y. Cell Commun Signal. 2024. PMID: 38863060 Free PMC article.
-
Idebenone attenuates RANKL-induced osteoclastogenesis and improves bone mass in ovariectomized mice.Free Radic Biol Med. 2025 Oct;238:206-219. doi: 10.1016/j.freeradbiomed.2025.06.043. Epub 2025 Jun 25. Free Radic Biol Med. 2025. PMID: 40578539
-
Licochalcone D inhibits osteoclast differentiation and postmenopausal osteoporosis by inactivating the NF-κB signaling pathway.J Orthop Surg Res. 2025 Jul 28;20(1):713. doi: 10.1186/s13018-025-06132-0. J Orthop Surg Res. 2025. PMID: 40722101 Free PMC article.
-
Glycogen Synthase Kinase-3 Beta (GSK3β) as a Potential Drug Target in Regulating Osteoclastogenesis: An Updated Review on Current Evidence.Biomolecules. 2024 Apr 21;14(4):502. doi: 10.3390/biom14040502. Biomolecules. 2024. PMID: 38672518 Free PMC article. Review.
-
Docosahexaenoic Acid Inhibits Osteoclastogenesis via FFAR4-Mediated Regulation of Inflammatory Cytokines.Molecules. 2025 Jul 29;30(15):3180. doi: 10.3390/molecules30153180. Molecules. 2025. PMID: 40807354 Free PMC article. Review.
Cited by
-
Correction: Yun et al. Rhusflavone Modulates Osteoclastogenesis Through RANKL-Induced AKT Signaling in Bone Marrow-Derived Macrophages. Int. J. Mol. Sci. 2025, 26, 3025.Int J Mol Sci. 2025 Jul 30;26(15):7354. doi: 10.3390/ijms26157354. Int J Mol Sci. 2025. PMID: 40806792 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

