Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability
- PMID: 40243703
- PMCID: PMC11988840
- DOI: 10.3390/ijms26073045
Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability
Abstract
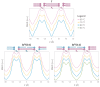
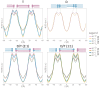
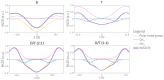
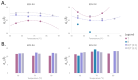
Archaeal membranes exhibit remarkable stability under extreme environmental conditions, a feature attributed to their unique lipid composition. While it is widely accepted that tetraether lipids confer structural integrity by forming monolayers, the role of bilayer-forming diether lipids in membrane stability remains unclear. Here, we demonstrate that incorporating diethers into archaeal-like lipid assemblies enhances membrane organization and adaptability under thermal stress. Using neutron diffraction, we show that membranes composed of mixed diethers and tetraethers exhibit greater structural order and stability compared to pure lipid systems. Contrary to expectations, monolayer-forming tetraethers alone display increased variability in lamellar spacing under fluctuating temperature and humidity, whereas mixed lipid membranes maintain a consistent architecture. Furthermore, neutron-scattering length density profiles reveal an unexpected density feature at the bilayer midplane, challenging conventional models of archaeal monolayer organization. These findings suggest that molecular diversity of lipid molecules, rather than tetraether dominance, plays a critical role in membrane auto-assembly, stability, and adaptability. Our results provide new insights into archaeal membrane adaptation strategies, with implications for the development of bioinspired, robust synthetic membranes for industrial and biomedical applications.
Keywords: Archaea; archaeal lipids; membrane biophysics; monolayer membrane; neutron diffraction; temperature stability; tetraether.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Characterisation of a synthetic Archeal membrane reveals a possible new adaptation route to extreme conditions.Commun Biol. 2021 Jun 2;4(1):653. doi: 10.1038/s42003-021-02178-y. Commun Biol. 2021. PMID: 34079059 Free PMC article.
-
Structural Characterization of an Archaeal Lipid Bilayer as a Function of Hydration and Temperature.Int J Mol Sci. 2020 Mar 6;21(5):1816. doi: 10.3390/ijms21051816. Int J Mol Sci. 2020. PMID: 32155764 Free PMC article.
-
Molecular dynamics study of bipolar tetraether lipid membranes.Biophys J. 2005 Nov;89(5):3195-202. doi: 10.1529/biophysj.105.060962. Epub 2005 Aug 12. Biophys J. 2005. PMID: 16100279 Free PMC article.
-
The essence of being extremophilic: the role of the unique archaeal membrane lipids.Extremophiles. 1998 Aug;2(3):163-70. doi: 10.1007/s007920050056. Extremophiles. 1998. PMID: 9783161 Review.
-
Archaeal tetraether lipids: unique structures and applications.Appl Biochem Biotechnol. 2002 Jan;97(1):45-62. doi: 10.1385/abab:97:1:45. Appl Biochem Biotechnol. 2002. PMID: 11900115 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

