Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development
- PMID: 40243704
- PMCID: PMC11989139
- DOI: 10.3390/ijms26073037
Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development
Abstract
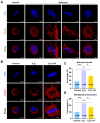
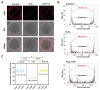
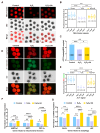
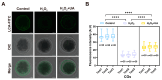
Both the livestock and biomedical fields require a large supply of high-quality mature oocytes. However, the in vitro maturation (IVM) process often leads to an accumulation of reactive oxygen species (ROS), which can cause defects in oocyte meiosis and embryo development, ultimately compromising oocyte quality. Urolithin A (UA), known for its antioxidant properties, has not been thoroughly investigated for its potential to mitigate the negative effects of oxidative stress during the in vitro culturing of oocytes, and its underlying mechanism is not well understood. In this study, an in vitro oxidative stress model was established using porcine oocytes treated with H2O2, followed by exposure to varying concentrations of UA. The results revealed that 30 μM UA significantly improved both the quality of oocyte culture and the developmental potential of the resulting embryos. UA was found to enhance oocyte autophagy, reduce oxidative stress-induced mitochondrial damage, and restore mitochondrial function. Additionally, it lowered ROS and DNA damage levels in the oocytes, maintained proper spindle/chromosome alignment and actin cytoskeleton structure, promoted nuclear maturation, prevented abnormal cortical granule distribution, and supported oocyte cytoplasmic maturation. As a result, UA alleviated oxidative stress-induced defects in oocyte maturation and cumulus cell expansion, thereby improving the developmental potential and quality of parthenogenetic embryos. After supplementation with UA, pig parthenogenetic embryo pluripotency-related genes (Nanog and Sox2) and antiapoptotic genes (Bcl2) were upregulated, while proapoptotic genes (Bax) were downregulated. In conclusion, this study suggests that adding UA during IVM can effectively mitigate the adverse effects of oxidative stress on porcine oocytes, presenting a promising strategy for enhancing their developmental potential in vitro.
Keywords: autophagy; embryo development; meiotic maturation; mitochondrial function; oocyte quality; oxidative stress; urolithin A.
Conflict of interest statement
The authors state that they have no potential conflicts of interest.
Figures





Similar articles
-
α-Ketoglutarate Improves Meiotic Maturation of Porcine Oocytes and Promotes the Development of PA Embryos, Potentially by Reducing Oxidative Stress through the Nrf2 Pathway.Oxid Med Cell Longev. 2022 Feb 21;2022:7113793. doi: 10.1155/2022/7113793. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35237383 Free PMC article.
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model.Hum Reprod. 2019 Oct 2;34(10):1984-1998. doi: 10.1093/humrep/dez161. Hum Reprod. 2019. PMID: 31625574
-
Could oxidative stress influence the in-vitro maturation of oocytes?Reprod Biomed Online. 2009 Jun;18(6):864-80. doi: 10.1016/s1472-6483(10)60038-7. Reprod Biomed Online. 2009. PMID: 19490793 Free PMC article. Review.
-
A Comprehensive Review of Mitochondrial Complex I During Mammalian Oocyte Maturation.Genesis. 2025 Jun;63(3):e70017. doi: 10.1002/dvg.70017. Genesis. 2025. PMID: 40452557 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

