Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair
- PMID: 40243729
- PMCID: PMC11988544
- DOI: 10.3390/ijms26073063
Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair
Erratum in
-
Correction: Almeida et al. Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair. Int. J. Mol. Sci. 2025, 26, 3063.Int J Mol Sci. 2025 Nov 10;26(22):10879. doi: 10.3390/ijms262210879. Int J Mol Sci. 2025. PMID: 41303723 Free PMC article.
Abstract
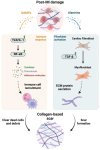
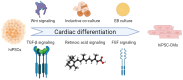
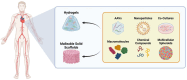
Cardiovascular disease (CVD) remains the leading cause of death globally, with myocardial infarction (MI) being a major contributor. The current therapeutic approaches are limited in effectively regenerating damaged cardiac tissue. Up-to-date strategies for heart regeneration/reconstitution aim at cardiac remodeling through repairing the damaged tissue with an external cell source or by stimulating the existing cells to proliferate and repopulate the compromised area. Cell reprogramming is addressed to this challenge as a promising solution, converting fibroblasts and other cell types into functional cardiomyocytes, either by reverting cells to a pluripotent state or by directly switching cell lineage. Several strategies such as gene editing and the application of miRNA and small molecules have been explored for their potential to enhance cardiac regeneration. Those strategies take advantage of cell plasticity by introducing reprogramming factors that regress cell maturity in vitro, allowing for their later differentiation and thus endorsing cell transplantation, or promote in situ cell proliferation, leveraged by scaffolds embedded with pro-regenerative factors promoting efficient heart restoration. Despite notable advancements, important challenges persist, including low reprogramming efficiency, cell maturation limitations, and safety concerns in clinical applications. Nonetheless, integrating these innovative approaches offers a promising alternative for restoring cardiac function and reducing the dependency on full heart transplants.
Keywords: cardiovascular diseases; dedifferentiation; direct reprogramming; indirect reprogramming; transdifferentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organization (WHO) Cardiovascular Diseases (CVDs) [(accessed on 3 April 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases....
-
- Thygesen K., Alpert J.S., White H.D. Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2007;50:2173–2195. - PubMed
-
- Malliaras K., Zhang Y., Seinfeld J., Galang G., Tseliou E., Cheng K., Sun B., Aminzadeh M., Marbán E. Cardiomyocyte Proliferation and Progenitor Cell Recruitment Underlie Therapeutic Regeneration after Myocardial Infarction in the Adult Mouse Heart. EMBO Mol. Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- PTDC/ BIMMED/3363/2014/Fundação para a Ciência e Tecnologia, Portugal
- UIDB/04462/2020 and UIDP/04462/2020/iNOVA4Health, Fundação para a Ciência e Tecnologia, Portugal
- LA/P/0087/2020/Associated Laboratory LS4FUTURE, Fundação para a Ciência e Tecnologia, Portugal
- 06890/2021/BD/Fundação para a Ciência e Tecnologia, Portugal
LinkOut - more resources
Full Text Sources

