Tekt3 Safeguards Proper Functions and Morphology of Neuromast Hair Bundles
- PMID: 40243732
- PMCID: PMC11989051
- DOI: 10.3390/ijms26073115
Tekt3 Safeguards Proper Functions and Morphology of Neuromast Hair Bundles
Abstract
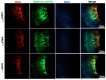
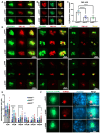
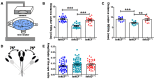
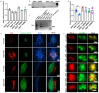
The inner ear and/or lateral line are responsible for hearing and balance of vertebrate. The otic sensory hair cells (HCs) employ cilium organelles, namely stereocilia and/or kinocilia, to mediate mechanical stimuli to electrical signal transition. Tektins (Tekts) are known as the cilium microtubule stabilizer and inner-space filler, and four Tekt(1-4)-encoding genes are identified in zebrafish HCs, but the subcellular location of Tekts in HCs remains unknown. In the present study, we first found that tekt3 is expressed in the inner ear and lateral line neuromast. Antibody staining revealed that Tekt3 is present in neuromast and utricular HCs. It is absent in the saccule, the authentic hearing end-organ of zebrafish and the crista of semi-circular canals. Furthermore, Tekt3 were enriched at the apical side of neuromast and utricular HCs, mainly in the cytosol. Similar subcellular distribution of Tekt3 was also evident in the outer HCs of mature mouse cochlea, which are not directly linked to the hearing sense. However, only neuromast HCs exerted morphological defect of kinocilia in tekt3 mutant. The disrupted or distorted HC kinocilia of mutant neuromast ultimately resulted in slower vital dye intake, delayed HC regeneration after neomycin treatment, and reduced startle response to vibration stimulation. All functional defects of tekt3 mutant were largely rescued by wild-type tekt3 mRNA. Our study thus suggests that zebrafish Tekt3 maintains the integrity and function of neuromast kinocilia to against surrounding and persistent low-frequency noises, perhaps via the intracellular distribution of Tekt3. Nevertheless, TEKT3/Tekt3 could be used to clarify HC sub-types in both zebrafish and mice, to highlight the non-hearing HCs.
Keywords: cilia; hair cell; regeneration; tekt3; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

