Recent Developments in the [1,2]-Phospha-Brook Rearrangement Reaction
- PMID: 40243753
- PMCID: PMC11989137
- DOI: 10.3390/ijms26073065
Recent Developments in the [1,2]-Phospha-Brook Rearrangement Reaction
Abstract
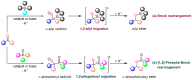
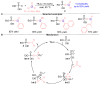
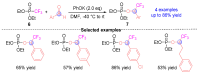
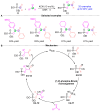
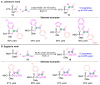
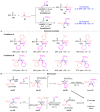
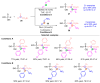
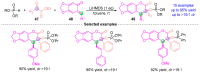
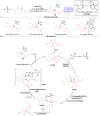
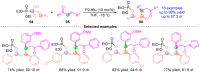
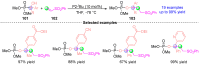
The [1,2]-phospha-Brook rearrangement serves as a powerful synthetic strategy that enables efficient carbonyl umpolung through phosphoryl group migration, providing direct access to α-hydroxyphosphoryl compounds-a privileged class of synthons with broad applications in organophosphorus chemistry, medicinal chemistry, and materials science. This review provides a comprehensive overview of recent progress in synthetic methodologies, possible mechanisms, and asymmetric transformations, highlighting key breakthroughs and future directions in this rapidly evolving field.
Keywords: [1,2]-phospha-Brook rearrangement; carbonyl compounds; phosphodiesters.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures































Similar articles
-
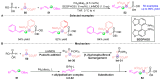
Efficient Synthesis of Polysubstituted Pyrroles Based on [3+2] Cycloaddition Strategy Utilizing [1,2]-Phospha-Brook Rearrangement under Brønsted Base Catalysis.Chemistry. 2018 Oct 12;24(57):15246-15253. doi: 10.1002/chem.201803809. Epub 2018 Oct 5. Chemistry. 2018. PMID: 30113749
-
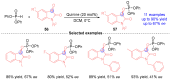
Catalytic Generation of Benzyl Anions from Aryl Ketones Utilizing [1,2]-Phospha-Brook Rearrangement and Their Application to Synthesis of Tertiary Benzylic Alcohols.Chemistry. 2024 Dec 10;30(69):e202402967. doi: 10.1002/chem.202402967. Epub 2024 Oct 16. Chemistry. 2024. PMID: 39215614
-
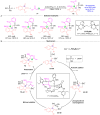
Novel Stereo-Induction Pattern in Pudovik Addition/Phospha-Brook Rearrangement Towards Chiral Trisubstituted Allenes.Angew Chem Int Ed Engl. 2024 May 27;63(22):e202403707. doi: 10.1002/anie.202403707. Epub 2024 Apr 12. Angew Chem Int Ed Engl. 2024. PMID: 38520267
-
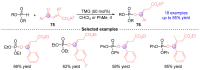
Stereoselective Reductive Coupling Reactions Utilizing [1,2]-Phospha-Brook Rearrangement: A Powerful Umpolung Approach.J Org Chem. 2023 Aug 4;88(15):10325-10338. doi: 10.1021/acs.joc.3c01055. Epub 2023 Jul 17. J Org Chem. 2023. PMID: 37460945 Review.
-
Radical Brook rearrangement: past, present, and future.Chem Soc Rev. 2025 Feb 17;54(4):1870-1904. doi: 10.1039/d4cs01275e. Chem Soc Rev. 2025. PMID: 39835385 Review.
References
-
- Brook A.G. Isomerism of Some α-Hydroxysilanes to Silyl Ethers. J. Am. Chem. Soc. 1958;80:1886–1889. doi: 10.1021/ja01541a026. - DOI
-
- Yang F., Wang J., Dong Y., Zhang N., Zhang C. Transition Metal Promoted Brook Rearrangement and Its Related Reactions. Tetrahedron. 2024;168:134351. doi: 10.1016/j.tet.2024.134351. - DOI
-
- Kondoh A., Terada M. Development of Molecular Transformations on the Basis of Catalytic Generation of Anionic Species by Organosuperbase. Bull. Chem. Soc. Jpn. 2021;94:339–356. doi: 10.1246/bcsj.20200308. - DOI
-
- Kondoh A., Terada M. [1,2]-Phospha-Brook Rearrangement as Tool for Generation of Anionic Nucleophiles in Addition Reactions under Bronsted Base Catalysis. Asian. J. Org. Chem. 2023;12:e202300003.
-
- Kaur R., Singh R.P. Stereoselective Reductive Coupling Reactions Utilizing 1,2-Phospha-Brook Rearrangement: A Powerful Umpolung Approach. J. Org. Chem. 2023;88:10325–10338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

