In Vivo Engineered CAR-T Cell Therapy: Lessons Built from COVID-19 mRNA Vaccines
- PMID: 40243757
- PMCID: PMC11988490
- DOI: 10.3390/ijms26073119
In Vivo Engineered CAR-T Cell Therapy: Lessons Built from COVID-19 mRNA Vaccines
Abstract
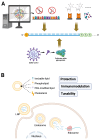
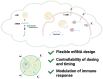
Chimeric antigen receptor T cell (CAR-T) therapy has revolutionized cancer immunotherapy but continues to face significant challenges that limit its broader application, such as antigen targeting, the tumor microenvironment, and cell persistence, especially in solid tumors. Meanwhile, the global implementation of mRNA vaccines during the COVID-19 pandemic has highlighted the transformative potential of mRNA and lipid nanoparticle (LNP) technologies. These innovations, characterized by their swift development timelines, precise antigen design, and efficient delivery mechanisms, provide a promising framework to address some limitations of CAR-T therapy. Recent advancements, including mRNA-based CAR engineering and optimized LNP delivery, have demonstrated the capacity to enhance CAR-T efficacy, particularly in the context of solid tumors. This review explores how mRNA-LNP technology can drive the development of in vivo engineered CAR-T therapies to address current limitations and discusses future directions, including advancements in mRNA design, LNP optimization, and strategies for improving in vivo CAR-T functionality and safety. By bridging these technological insights, CAR-T therapy may evolve into a versatile and accessible treatment paradigm across diverse oncological landscapes.
Keywords: CAR-T therapy; immune response regulation; lipid nanoparticles; mRNA technology; mRNA vaccines.
Conflict of interest statement
Partial institutional endowments were received from Hirotsu Bio Science Inc. (Tokyo, Japan); Kinshu-kai Medical Corporation (Osaka, Japan); Kyowa-kai Medical Corporation (Osaka, Japan); IDEA Consultants Inc. (Tokyo, Japan); and Unitech Co., Ltd. (Chiba, Japan), to Department of Medical Data Science, Center of Medical Innovation and Translational Research, Osaka University Graduate School of Medicine, Yamadaoka 2-2, Suita 565-0871, Osaka, Japan. H. I. has a fixed-term employment contract with Kyowa-kai Medical Corporation (from March, 2024, through August, 2024). Others have no conflict of interest for this study.
Figures
References
-
- Brudno J.N., Maric I., Hartman S.D., Rose J.J., Wang M., Lam N., Stetler-Stevenson M., Salem D., Yuan C., Pavletic S., et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018;36:2267–2280. doi: 10.1200/jco.2018.77.8084. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- grant nos. 19K22658, 20H00541, 21K19526, 22H03146, 22K19559, 23K19505, 23K18313, 23KK0153, 24K22144, and 16H06279 (PAGS)/Ministry of Education, Culture, Sports, Science and Technology
- grant nos. JP23ym0126809 and JP24ym0126809/Japan Agency for Medical Research and Development
- 23-255001/Princess Takamatsu Cancer Research Fund
- G-2024-3-00/IFO Research Communications
- 2024/Oceanic Wellness Foundation
LinkOut - more resources
Full Text Sources
Medical

