Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism
- PMID: 40243802
- PMCID: PMC11988970
- DOI: 10.3390/ijms26073094
Molecular Mechanism of Microgravity-Induced Intestinal Flora Dysbiosis on the Abnormalities of Liver and Brain Metabolism
Abstract
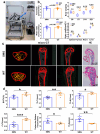
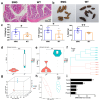
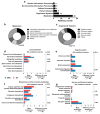
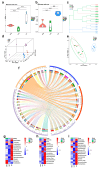
Space flight has many adverse effects on the physiological functions of astronauts. Certain similarities have been observed in some physiological processes of rodents and astronauts in space, although there are also differences. These similarities make rodents helpful models for initial investigations into space-induced physiological changes. This study uses a 3D-Clinostat to simulate microgravity and explores the role of microgravity in space flight-induced liver and brain abnormalities by comparing changes in the gut microbiota, serum metabolites, and the function and physiological biochemistry of liver and brain tissues between the simulated microgravity (SMG) group mice and the wild type (WT) group mice. The study, based on hematoxylin-eosin (HE) staining, 16S sequencing technology, and non-targeted metabolomics analysis, shows that the gut tissue morphology of the SMG group mice is abnormal, and the structure of the gut microbiota and the serum metabolite profile are imbalanced. Furthermore, using PICRUST 2 technology, we have predicted the functions of the gut microbiota and serum metabolites, and the results indicate that the liver metabolism and functions (including lipid metabolism, amino acid metabolism, and sugar metabolism, etc.) of the SMG group mice are disrupted, and the brain tissue metabolism and functions (including neurotransmitters and hormone secretion, etc.) are abnormal, suggesting a close relationship between microgravity and liver metabolic dysfunction and brain dysfunction. Additionally, the high similarity in the structure of the gut microbiota and serum metabolite profile between the fecal microbiota transplant (FMT) group mice and the SMG group mice, and the physiological and biochemical differences in liver and brain tissues compared to the WT group mice, suggest that microgravity induces imbalances in the gut microbiota, which in turn triggers abnormalities in liver and brain metabolism and function. Finally, through MetaMapp analysis and Pearson correlation analysis, we found that valeric acid, a metabolite of gut microbiota, is more likely to be the key metabolite that relates to microgravity-induced gut microbiota abnormalities, disorders of amino acid and lipid metabolism, and further induced metabolic or functional disorders in the liver and brain. This study has significant practical application value for deepening the understanding of the adaptability of living organisms in the space environment.
Keywords: 3D-Clinostat; brain; gut microbiota; liver; metabolic dysfunction; microgravity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Simulated microgravity suppresses MAPK pathway-mediated innate immune response to bacterial infection and induces gut microbiota dysbiosis.FASEB J. 2020 Nov;34(11):14631-14644. doi: 10.1096/fj.202001428R. Epub 2020 Sep 12. FASEB J. 2020. PMID: 32918764
-
Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model.FASEB J. 2019 Sep;33(9):10140-10151. doi: 10.1096/fj.201900238RR. Epub 2019 Jun 25. FASEB J. 2019. PMID: 31238017
-
Behavioral and multiomics analysis of 3D clinostat simulated microgravity effect in mice focusing on the central nervous system.Sci Rep. 2025 Feb 17;15(1):5731. doi: 10.1038/s41598-025-90212-y. Sci Rep. 2025. PMID: 39962314 Free PMC article.
-
Gut microbiome and human health under the space environment.J Appl Microbiol. 2021 Jan;130(1):14-24. doi: 10.1111/jam.14789. Epub 2020 Aug 10. J Appl Microbiol. 2021. PMID: 32692438 Review.
-
Cardiovascular changes under the microgravity environment and the gut microbiome.Life Sci Space Res (Amst). 2024 Feb;40:89-96. doi: 10.1016/j.lssr.2023.09.003. Epub 2023 Sep 9. Life Sci Space Res (Amst). 2024. PMID: 38245353 Review.
References
-
- Sisovas L., Ceponis A., Borodinas S. The Challenges of Piezoelectric Actuators and Motors Application in a Space Environment. Actuators. 2024;13:312. doi: 10.3390/act13080312. - DOI
-
- Drago-Ferrante R., Di Fiore R., Karouia F., Subbannayya Y., Das S., Mathyk B.A., Arif S., Guevara-Cerdán A.P., Seylani A., Galsinh A.S., et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. Int. J. Mol. Sci. 2022;23:7465. doi: 10.3390/ijms23137465. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

