Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for Crohn's disease and ulcerative colitis: 2-year results from open-label extensions of two randomized controlled trials (LIBERTY)
- PMID: 40243842
- PMCID: PMC12134888
- DOI: 10.1093/ecco-jcc/jjaf060
Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for Crohn's disease and ulcerative colitis: 2-year results from open-label extensions of two randomized controlled trials (LIBERTY)
Abstract
Background and aims: In the LIBERTY phase 3 studies in Crohn's disease (CD) or ulcerative colitis (UC), maintenance CT-P13 subcutaneous (SC) 120 mg was more effective than placebo after 1 year. Here we report 2-year data from the LIBERTY open-label extensions.
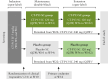
Methods: Two randomized, placebo-controlled, double-blind studies evaluated the efficacy and safety of CT-P13 SC maintenance in moderate-to-severe CD or UC. Responders to CT-P13 intravenous induction were randomized at week (W) 10 to CT-P13 SC 120 mg or placebo biweekly, until W54. From W22, dose adjustment to CT-P13 SC 240 mg was permitted for loss of response. At W56, patients could enter an open-label extension, receiving CT-P13 SC 120 mg (or 240 mg if dose-adjusted), biweekly, until W102.
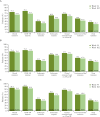
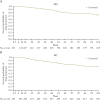
Results: The extension comprised 278/343 (81.0%) and 348/438 (79.5%) patients in the CD and UC studies, respectively. In those continuing on-study, efficacy (non-responder imputation) was well maintained in the CT-P13 SC group at W102: 63.5% (as-observed: 70.5%) and 49.0% (as-observed: 58.8%) of CD patients (N = 192) achieved clinical remission and endoscopic response, respectively; 45.1% (as-observed: 60.1%) and 41.4% (as-observed: 52.4%) of UC patients (N = 237) achieved clinical remission and endoscopic-histologic mucosal improvement, respectively. No new safety signals were identified from longer-term CT-P13 SC treatment or starting CT-P13 SC 120 mg after placebo, with similar adverse event rates for patients undergoing dose adjustment to CT-P13 SC 240 mg from CT-P13 SC 120 mg or placebo.
Conclusion: CT-P13 SC is an effective and well-tolerated long-term maintenance treatment in moderate-to-severe CD and UC.
Clinicaltrials.gov identifiers: NCT03945019 (CD) and NCT04205643 (UC).
Keywords: CT-P13 SC; LIBERTY; inflammatory bowel disease.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
J.-.F.C. reports receiving research grants from AbbVie, Bristol Myers Squibb, Janssen Pharmaceuticals, Prometheus, and Takeda; receiving payment for lectures from AbbVie and Takeda; receiving consulting fees from AbbVie, Allergan, Amgen, AnaptysBio, Arena Pharmaceuticals, Astellas, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly, Envision Pharma Group, Ferring Pharmaceuticals, Galmed Research, Genentech (Roche), GlaxoSmithKline, Immunic, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos Biopharma, Merck, Microba Life Sciences, Novartis, Otsuka Pharmaceutical, Pfizer, Protagonist Therapeutics, Sanofi, Sun Pharma, Takeda, TiGenix, and Vifor; and holding stock options in Intestinal Biotech Development.
W.J.S. reports receiving research grants from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Pfizer, Prometheus Laboratories, Seres Therapeutics, Shire Pharmaceuticals, Takeda, and Theravance Biopharma; receiving consulting fees from AbbVie, Abivax, Admirx (now Q32 Bio), Alfasigma, Alimentiv, Alivio Therapeutics, Allakos, Amgen, Arena Pharmaceuticals, AstraZeneca, Atlantic Pharmaceuticals, Bausch Health (Salix), BeiGene, Bellatrix Pharmaceuticals, Biora (Progenity), Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Celgene, Celltrion, ClostraBio, Codexis, Eli Lilly, Equillium, Forbion, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Immunic (Vital Therapies), Index Pharmaceuticals, Inotrem, Intact Therapeutics, Iota Biosciences, Janssen Pharmaceuticals, Kiniksa Pharmaceuticals, Kyverna Therapeutics, Landos Biopharma, Morphic Therapeutics, Novartis, Ono Pharmaceuticals, Oppilan Pharma (now Ventyx Biosciences), Otsuka Pharmaceutical, Pandion Therapeutics, Pfizer, Pharm Olam, Polpharma, Prometheus Biosciences, Protagonist Therapeutics, PTM Therapeutics, Quell Therapeutics, Reistone Biopharma, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tillotts Pharma, Vedanta Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vividion Therapeutics, Vivreon Gastrosciences, Xencor, and Zealand Pharma; receiving stock or stock options from Allakos, BeiGene, Biora (Progenity), Gossamer Bio, Oppilan Pharma (now Ventyx Biosciences), Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Gastrosciences; and employment by Shoreline Biosciences and Ventyx Biosciences. W.J.S. also reports that their spouse has received consulting fees from Iveric Bio and Prometheus Laboratories; has received stock or stock options from Oppilan Pharma (now Ventyx Biosciences), Progenity, Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, and Vimalan Biosciences; and has been an employee of Prometheus Biosciences.
S.Sc. reports receiving personal fees for consulting in advisory boards from AbbVie, Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Biogen, Celltrion, Celgene, Dr Falk Pharma, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, IMAB, MSD, Mylan, Pfizer, Fresenius, Janssen Pharmaceuticals, Takeda, Theravance Biopharma, Provention Bio, Protagonist Therapeutics, Sandoz/Hexal, and UCB.
S.D. reports receiving consulting fees from AbbVie, Alimentiv, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring Pharmaceuticals, Gilead Sciences, Hospira, Inotrem, Janssen Pharmaceuticals, Johnson & Johnson, Morphic Therapeutics, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, Teladoc Health, TiGenix, UCB, Vial, and Vifor; and receiving speaker honoraria from AbbVie, Amgen, Ferring Pharmaceuticals, Gilead Sciences, Janssen Pharmaceuticals, Mylan, Pfizer, and Takeda.
M.Kł. reports receiving clinical trial support from Celltrion; receiving consulting fees, payment for lectures, and support for attending congresses from Takeda; receiving lecture fees from Janssen Pharmaceuticals and Ferring Pharmaceuticals; and advisory board membership for Bristol Myers Squibb and Eli Lilly.
S.Sr. reports receiving personal fees for consulting in advisory boards from AbbVie, Amgen, Arena Pharmaceuticals, Bristol Myers Squibb, Biogen, Celltrion, Celgene, Dr Falk Pharma, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, IMAB, MSD, Mylan, Pfizer, Fresenius, Janssen Pharmaceuticals, Provention Bio, Protagonist, Takeda, Theravance Biopharma, Sandoz/Hexal, and UCB.
A.L. reports receiving speaker honoraria from AbbVie, Bristol Myers Squibb, Eli Lilly, Janssen Pharmaceuticals, Pfizer, and Takeda; and receiving fees for participation on a data safety monitoring board or advisory board from AbbVie and Takeda.
A.G. reports receiving consulting fees from AbbVie, Malesci, MSD, Roche, Sanofi, and Takeda.
S.J.L., S.K., Y.B., Su.L., Se.L., J.M.K., and G.P. are employees of Celltrion and have received stock or stock options from Celltrion.
J.H.L., Ji.L., Ju.L., and J.Y.R. are employees of Celltrion.
B.E.S. reports receiving consulting fees from AbbVie, Alimentiv, Amgen, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Boehringer Ingelheim, Boston Pharmaceuticals, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Fresenius, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Kaleido Biosciences, Kallyope, Merck, Morphic Therapeutics, MRM Health, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Sun Pharma, Surrozen, Target RWE, Teva, TLL Pharmaceutical, and Ventyx Biosciences; receiving consulting and speaking fees from Abivax; receiving consulting and speaking fees and other support from Eli Lilly; receiving research grants, consulting and speaking fees, and other support from Bristol Myers Squibb, Janssen Pharmaceuticals, Pfizer, and Takeda; receiving research grants and consulting fees from Theravance Biopharma; and receiving stock options from Ventyx Biosciences.
The following authors report that they have no conflicts of interest to declare: J.K., R.K., M.G., A.S., P.S., E.V., M.H., M.O., V.B., M.Ko., D.S., R.S., and S.B.H.
Figures



References
-
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

