Archaeal Lipids: Extraction, Separation, and Identification via Natural Product Chemistry Perspective
- PMID: 40243902
- PMCID: PMC11988811
- DOI: 10.3390/ijms26073167
Archaeal Lipids: Extraction, Separation, and Identification via Natural Product Chemistry Perspective
Abstract
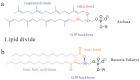
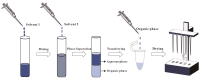
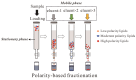
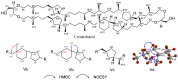
Archaeal lipids, defining a primordial life domain alongside Bacteria and Eukarya, are distinguished by their unique glycerol-1-phosphate backbone and ether-linked isoprenoid chains. Serving as critical geochemical biomarkers, archaeal lipids like glycerol dialkyl glycerol tetraethers (GDGTs) underpin paleoclimate proxies, while their phylum-specific distributions illuminate phylogenetic divergence. Despite the maturity of Mass Spectrometry-based quantitative biomarkers-predominantly those with established structures-becoming well-established in geochemical research, systematic investigation of archaeal lipids as natural products has notably lagged. This deficit manifests across three key dimensions: (1) Extraction methodology lacks universal protocols adapted to diverse archaeal taxa and sample matrices. While comparative studies exist, theoretical frameworks guiding method selection remain underexplored. (2) Purification challenges persist due to the unique structures and complex isomerization profiles of archaeal lipids, hindering standardized separation protocols. (3) Most critically, structural characterization predominantly depends on decades-old foundational studies. However, the existing reviews prioritize chemical structural, biosynthetic, and applied aspects of archaeal lipids over analytical workflows. This review addresses this gap by adopting a natural product chemistry perspective, integrating three key aspects: (1) the clarification of applicable objects, scopes, and methodological mechanisms of various extraction technologies for archaeal lipids, encompassing both cultured and environmental samples; (2) the elucidation of separation principles underlying polar-gradient lipid fractionation processes, leveraging advanced chromatographic technologies; (3) the detailed exploration of applications for NMR in resolving complex lipid structures, with specialized emphasis on determining the stereochemical configuration. By synthesizing six decades of methodological evolution, we establish a comprehensive analytical framework, from lipids extraction to structural identification. This integrated approach constructs a systematic methodological paradigm for archaeal lipid analysis, bridging theoretical principles with practical implementation.
Keywords: archaea; extraction; identification; lipids; natural product chemistry; separation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Environmental factors shaping the archaeal community structure and ether lipid distribution in a subtropic river and estuary, China.Appl Microbiol Biotechnol. 2018 Jan;102(1):461-474. doi: 10.1007/s00253-017-8595-8. Epub 2017 Nov 4. Appl Microbiol Biotechnol. 2018. PMID: 29103169
-
Glycerol monoalkanediol diethers: a novel series of archaeal lipids detected in hydrothermal environments.Rapid Commun Mass Spectrom. 2016 Jan 15;30(1):54-60. doi: 10.1002/rcm.7417. Rapid Commun Mass Spectrom. 2016. PMID: 26661970
-
A combined lipidomic and 16S rRNA gene amplicon sequencing approach reveals archaeal sources of intact polar lipids in the stratified Black Sea water column.Geobiology. 2019 Jan;17(1):91-109. doi: 10.1111/gbi.12316. Epub 2018 Oct 3. Geobiology. 2019. PMID: 30281902 Free PMC article.
-
Biosynthesis of archaeal membrane ether lipids.Front Microbiol. 2014 Nov 26;5:641. doi: 10.3389/fmicb.2014.00641. eCollection 2014. Front Microbiol. 2014. PMID: 25505460 Free PMC article. Review.
-
Archaeal phospholipids: Structural properties and biosynthesis.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Nov;1862(11):1325-1339. doi: 10.1016/j.bbalip.2016.12.006. Epub 2016 Dec 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28007654 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 32201201/National Natural Science Foundation of China
- 2060302/Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources, grant number
- 2024JH2/102600068/Liaoning Provincial Joint Science and Technology Plan (Key Technology Research and Development Project)
- 2024RY011/Dalian Excellent Young Scientific and Technological Talent Project
- 202301YB04/Dalian University Research Platform Project
LinkOut - more resources
Full Text Sources
Miscellaneous

