Integrins in Cancer Drug Resistance: Molecular Mechanisms and Clinical Implications
- PMID: 40243917
- PMCID: PMC11989024
- DOI: 10.3390/ijms26073143
Integrins in Cancer Drug Resistance: Molecular Mechanisms and Clinical Implications
Abstract
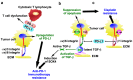
It is estimated that between 80 and 90% of mortality in cancer patients is directly or indirectly related to drug resistance. Consequently, overcoming drug resistance represents a significant challenge in the treatment of cancer. Integrins are transmembrane adhesion molecules that facilitate the linkage between the extracellular matrix (ECM) and the cytoskeleton, thereby enabling the activation of various cellular signaling pathways. Integrins are highly expressed in various cancers and contribute to cancer progression through invasion and metastasis. In addition, recent studies have revealed that integrins play a pivotal role in the development of drug resistance in cancer. This review will first provide an overview of integrin function and classification. It then discusses recent advances in understanding how integrins contribute to drug resistance in cancer, with a focus on ECM, drug transporters, the epithelial-to-mesenchymal transition (EMT), cancer stemness, PD-L1, and glycosylation. Finally, the potential applications of integrins as targets for therapeutic agents against drug-resistant cancers are also summarized.
Keywords: PD-L1; cancer; cancer stem cell (CSC); drug resistance; drug transporter; epithelial-to-mesenchymal transition (EMT); extracellular matrix (ECM); glycosylation; integrin; tyrosine kinase inhibitor (TKI).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Extracellular matrix as a contextual determinant of transforming growth factor-β signaling in epithelial-mesenchymal transition and in cancer.Cell Adh Migr. 2014;8(6):588-94. doi: 10.4161/19336918.2014.972788. Cell Adh Migr. 2014. PMID: 25482625 Free PMC article. Review.
-
Role of RGD-binding Integrins in ovarian cancer progression, metastasis and response to therapy.Integr Biol (Camb). 2025 Jan 8;17:zyaf003. doi: 10.1093/intbio/zyaf003. Integr Biol (Camb). 2025. PMID: 39916547 Review.
-
Integrins regulate stemness in solid tumor: an emerging therapeutic target.J Hematol Oncol. 2021 Oct 29;14(1):177. doi: 10.1186/s13045-021-01192-1. J Hematol Oncol. 2021. PMID: 34715893 Free PMC article. Review.
-
Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism.Cancer Res. 1996 Dec 1;56(23):5309-18. Cancer Res. 1996. PMID: 8968075 Review.
-
Exploiting Matrix Stiffness to Overcome Drug Resistance.ACS Biomater Sci Eng. 2024 Aug 12;10(8):4682-4700. doi: 10.1021/acsbiomaterials.4c00445. Epub 2024 Jul 5. ACS Biomater Sci Eng. 2024. PMID: 38967485 Free PMC article. Review.
Cited by
-
Utility of Multicellular Spheroids for Investigating Mechanisms of Chemoresistance in Triple-Negative Breast Cancer.Int J Mol Sci. 2025 Aug 3;26(15):7503. doi: 10.3390/ijms26157503. Int J Mol Sci. 2025. PMID: 40806629 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

