Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance
- PMID: 40243930
- PMCID: PMC11989004
- DOI: 10.3390/ijms26073174
Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance
Abstract
In recent years, the interest in developing genetically modified microorganisms (GMMs), including GMMs developed by genome editing, for use in the environment has significantly increased. However, the scientific knowledge on the ecology of such GMMs is severely limited. There is also little experience at the hands of regulators on how to evaluate the environmental safety of GMMs and on how to assess whether they provide sustainable alternatives to current (agricultural) production systems. This review analyzes two different GMM applications, GM microalgae for biofuel production and nitrogen-fixing GM soil bacteria for use as biofertilizers. We assess the challenges posed by such GMMs for regulatory environmental risk assessment (ERA) against the background of the GMO legislation existing in the European Union (EU). Based on our analysis, we present recommendations for ERA and the monitoring of GMM applications, and in particular for the improvement of the existing EU guidance. We also explore whether existing approaches for technology assessment can provide a framework for the broader assessment of GMM applications. To this end, we recommend developing and implementing an evidence-based sustainability analysis and other methods of technology assessment to support decision making and to address broader societal concerns linked to the use of GMM applications in the environment.
Keywords: ERA; GM biofertilizer; GM microalgae; GMM; TA; biosafety; environmental risk assessment; genetically modified microorganisms; governance; monitoring; sustainability assessment; technology assessment.
Conflict of interest statement
M.E., K.H. and M.O. are employed by the institution that commissioned this study. The retaining authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


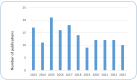
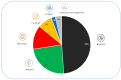
Similar articles
-
Principles for the risk assessment of genetically modified microorganisms and their food products in the European Union.Int J Food Microbiol. 2013 Oct 1;167(1):2-7. doi: 10.1016/j.ijfoodmicro.2013.03.013. Epub 2013 Mar 23. Int J Food Microbiol. 2013. PMID: 23632210
-
Design and regulation of engineered bacteria for environmental release.Nat Microbiol. 2025 Feb;10(2):281-300. doi: 10.1038/s41564-024-01918-0. Epub 2025 Feb 4. Nat Microbiol. 2025. PMID: 39905169 Review.
-
EFSA's scientific activities and achievements on the risk assessment of genetically modified organisms (GMOs) during its first decade of existence: looking back and ahead.Transgenic Res. 2014 Feb;23(1):1-25. doi: 10.1007/s11248-013-9741-4. Epub 2013 Aug 21. Transgenic Res. 2014. PMID: 23963741 Review.
-
Long term evaluation of field-released genetically modified rhizobia.Environ Biosafety Res. 2007 Jul-Sep;6(3):167-81. doi: 10.1051/ebr:2007006. Epub 2007 Jul 6. Environ Biosafety Res. 2007. PMID: 18001684
-
Scanning the Horizon for Environmental Applications of Genetically Modified Viruses Reveals Challenges for Their Environmental Risk Assessment.Int J Mol Sci. 2024 Jan 25;25(3):1507. doi: 10.3390/ijms25031507. Int J Mol Sci. 2024. PMID: 38338787 Free PMC article. Review.
References
-
- Jackson D.A., Symons R.H., Berg P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: Circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1972;69:2904–2909. doi: 10.1073/pnas.69.10.2904. - DOI - PMC - PubMed
-
- Secretariat of the Convention on Biological Diversity Report of the Multidisciplinary Ad Hoc Technical Expert Group on Synthetic Biology to Support the Process for Broad and Regular Horizon Scanning, Monitoring and Assessment on Its Second Meeting. 2024. [(accessed on 27 November 2024)]. CBD/SYNBIO/AHTEG/2024/1/3; Montreal, QC, Canada. Available online: https://www.cbd.int/doc/c/a26e/a6dc/571dd825eb08eef3865f85de/synbio-ahte....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

