Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization
- PMID: 40243939
- PMCID: PMC11989042
- DOI: 10.3390/ijms26073144
Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization
Abstract
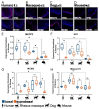
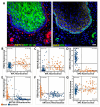
The mechanisms that influence human longevity are complex and operate on cellular, tissue, and organismal levels. To better understand the tissue-level mechanisms, we compared the organization of cell proliferation, differentiation, and cytoprotective protein expression in the squamous epithelium of the esophagus between mammals with varying lifespans. Humans are the only species with a quiescent basal stem cell layer that is distinctly physically separated from parabasal transit-amplifying cells. In addition to these stark differences in the organization of proliferation, human squamous epithelial stem cells express DNA repair-related markers, such as MECP2 and XPC, which are absent or low in mouse basal cells. Furthermore, we investigated whether the transition from basal to suprabasal is different between species. In humans, the parabasal cells seem to originate from cells detaching from the basement membrane, and these can already begin to proliferate while delaminating. In most other species, delaminating cells have been rare or their proliferation rate is different from that of their human counterparts, indicating an alternative mode of how stem cells maintain the tissue. In humans, the combination of an elevated cytoprotective signature and novel tissue organization may enhance resistance to aging and prevent cancer. Our results point to enhanced cellular cytoprotection and a tissue architecture which separates stemness and proliferation. These are both potential factors contributing to the increased fitness of human squamous epithelia to support longevity by suppressing tumorigenesis. However, the organization of canine oral mucosa shows some similarities to that of human tissue and may provide a useful model to understand the relationship between tissue architecture, gene expression regulation, tumor suppression, and longevity.
Keywords: MECP2; XPC; comparative biology; esophageal stem cells; multiplex immunofluorescence; spatial biology; squamous epithelial biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Association of TGFβ signaling with the maintenance of a quiescent stem cell niche in human oral mucosa.Histochem Cell Biol. 2016 Nov;146(5):539-555. doi: 10.1007/s00418-016-1473-0. Epub 2016 Aug 2. Histochem Cell Biol. 2016. PMID: 27480259
-
A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification.J Clin Invest. 2008 Dec;118(12):3860-9. doi: 10.1172/JCI35012. Epub 2008 Nov 6. J Clin Invest. 2008. PMID: 19033657 Free PMC article.
-
Overexpression of Kruppel-like factor 5 in esophageal epithelia in vivo leads to increased proliferation in basal but not suprabasal cells.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1784-92. doi: 10.1152/ajpgi.00541.2006. Epub 2007 Mar 29. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17395897
-
Divide and conquer: two stem cell populations in squamous epithelia, reserves and the active duty forces.Int J Oral Sci. 2019 Aug 27;11(3):26. doi: 10.1038/s41368-019-0061-2. Int J Oral Sci. 2019. PMID: 31451683 Free PMC article. Review.
-
Mesenchymal influences on epithelial differentiation in developing systems.J Cell Sci Suppl. 1988;10:195-230. doi: 10.1242/jcs.1988.supplement_10.15. J Cell Sci Suppl. 1988. PMID: 3077937 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

