Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation
- PMID: 40244012
- PMCID: PMC11989326
- DOI: 10.3390/ijms26073205
Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation
Abstract
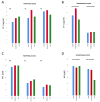
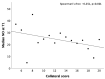
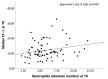
Good leptomeningeal collaterals (LMCs) after large vessel occlusion (LVO) extend the time window for endovascular therapy. The mechanisms regulating LMC activation are not fully understood. The aim of this study was to investigate the potential role of two vasoactive molecules endothelin-1 (ET-1)-a vasoconstrictor agent-and nitric oxide (NO)-a vasodilator agent-in the regulation of post-stroke LMCs. Ischemic stroke patients within 6 h of LVO were included. Collateral status was assessed using the Menon scoring system based on computed tomography angiography scans. Patients were accordingly divided into three groups: poor, intermediate, and good LMCs. Recanalization was evaluated using the modified thrombolysis in cerebral infarction (mTICI) score. Serum levels of ET-1 and NO were measured at three time points: T0 (<6 h), T1 (24 h), and T2 (48 h). A total of 105 patients were enrolled (mean age 76 ± 12.8 years): 44 with good (46.2%), 36 with intermediate (37.8%), and 22 with poor LMCs (23.1%). NO values decreased, whereas ET-1 values increased from T0 to T1 in all groups of patients. No significant association was found between serum ET-1 levels and collateral status. Higher ET-1 levels at T1 correlated with poor outcome regardless of the LMC status or the degree of recanalization (p = 0.030). A significant linear positive correlation was revealed at T0 between high levels of ET-1 and the neutrophil count (Spearman's rho = 0.236, p = 0.035). Subgroup analysis showed a significant inverse correlation at T1 between NO and the collateral score (Spearman's rho = -0.251, p = 0.021). Although we observed no significant association between LMC score and serum ET-1 concentrations, at 24 h higher ET-1 serum levels were predictive of poor outcome and higher NO levels were correlated with poor collateral status. These findings may indicate an inadequate microvascular reperfusion, possibly due to ET-1-mediated vasoconstriction, neutrophil activation, and NO-mediated oxidative stress, suggesting their potential role in the no-reflow phenomenon.
Keywords: ET-1; NO; acute ischemic stroke; leptomeningeal collaterals; reperfusion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Albers G.W., Marks M.P., Kemp S., Christensen S., Tsai J.P., Ortega-Gutierrez S., McTaggart R.A., Torbey M.T., Kim-Tenser M., Leslie-Mazwi T., et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

