Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands
- PMID: 40244013
- PMCID: PMC11989707
- DOI: 10.3390/ijms26073202
Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands
Abstract
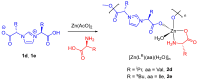
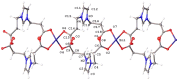
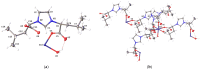
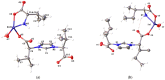
Coordination polymers containing zinc and imidazolium-based dicarboxylate ligands, [LR]-, were synthesized by reacting zinc acetate with HLR compounds, 1. The resulting complexes were characterized and structurally identified using single-crystal X-ray diffraction, revealing polymeric structures for the complexes [Zn(LR)2]n (R = Gly, 2a; βAla, 2b) and [Zn(LLeu)2(H2O)2]n (2c). In these structures, the [LR]- ligands adopt a bridging monodentate μ-κ1-O1,κ1-O3 coordination mode, resulting in distorted tetrahedral (2a, 2b) or octahedral (2c) geometries around the zinc center. When the synthesis was carried out in the presence of amino acids, mixed ligand complexes [Zn(LR)(aa)(H2O)]n (R = aa = Val, 2d, and R = aa = Ile, 2e) were formed. Complexes 2d-2e were also structurally characterized using single-crystal X-ray crystallography, revealing that the ligand [LR]- maintained the same coordination mode, while the zinc center adopted a five-coordinated geometry. The cytotoxic activity of complexes 2a-2e was evaluated against three cancer cell lines and one non-cancerous cell line. Remarkably, these complexes exhibited higher toxicity against cancer cells than against the non-cancerous cell line, and they showed greater selectivity than carboplatin, a commonly used chemotherapy drug. Although, in general, these complexes did not surpass the selectivity of gemcitabine, complex 2c stood out for exhibiting a selectivity index value similar to that of gemcitabine against melanoma cells. Among the series, compounds 2a-2c demonstrated the highest activity, with 2a being the only complex with some selective activity against lung cancer. Complex 2b was the most active, though with low selectivity, while complex 2c exhibited the highest selectivity for melanoma and bladder cancer (selectivity index of 3.0).
Keywords: X-ray; amino acid; anticancer; imidazolium-dicarboxylate; selectivity; zinc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Crystal structures, spectroscopic properties of new cobalt(II), nickel(II), zinc(II) and palladium(II) complexes derived from 2-acetyl-5-chloro thiophene thiosemicarbazone: Anticancer evaluation.Mater Sci Eng C Mater Biol Appl. 2019 May;98:550-559. doi: 10.1016/j.msec.2018.12.080. Epub 2018 Dec 30. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30813058
-
Synthesis, Characterization, and in Vitro Anticancer Activity of Copper and Zinc Bis(Thiosemicarbazone) Complexes.Inorg Chem. 2019 Oct 21;58(20):13709-13723. doi: 10.1021/acs.inorgchem.9b01281. Epub 2019 Jul 24. Inorg Chem. 2019. PMID: 31339305
-
[Zn(phen)(O,N,O)(H2O)] and [Zn(phen)(O,N)(H2O)] with O,N,O is 2,6-dipicolinate and N,O is L-threoninate: synthesis, characterization, and biomedical properties.J Biol Inorg Chem. 2012 Oct;17(7):1093-105. doi: 10.1007/s00775-012-0923-y. Epub 2012 Jul 24. J Biol Inorg Chem. 2012. PMID: 22825726
-
Pyrazolone-Based Zn(II) Complexes Display Antitumor Effects in Mutant p53-Carrying Cancer Cells.J Med Chem. 2024 Sep 12;67(17):15676-15690. doi: 10.1021/acs.jmedchem.4c01298. Epub 2024 Sep 2. J Med Chem. 2024. PMID: 39221914
-
Zn(II)-Curcumin Complexes-Based Anticancer Agents.ChemMedChem. 2024 Dec 2;19(23):e202400558. doi: 10.1002/cmdc.202400558. Epub 2024 Oct 29. ChemMedChem. 2024. PMID: 39225342 Review.
References
-
- Ott I. On the Medicinal Chemistry of Gold Complexes as Anticancer Drugs. Coord. Chem. Rev. 2009;253:1670–1681. doi: 10.1016/j.ccr.2009.02.019. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

