Strategies for Altering Delivery Technologies to Optimize CAR Therapy
- PMID: 40244018
- PMCID: PMC11989270
- DOI: 10.3390/ijms26073206
Strategies for Altering Delivery Technologies to Optimize CAR Therapy
Abstract
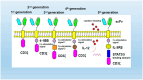
Chimeric antigen receptor (CAR) T-cell therapy has been proven to be an effective strategy for the treatment of hematological malignancies. At present, how to prepare CAR-T cells efficiently, quickly, and safely is one of the urgent problems to be solved. The durability and activity of engineered T cells in solid tumors need to be further improved, and the strategy of T cells penetrating the tumor microenvironment also needs to be improved. In addition, although the problems mainly caused by T-cell biology are being solved, the manufacturing mode and process still need to be improved to ensure that CAR-T cell therapy can be widely used. This paper summarizes some strategies that can improve the efficacy of CAR-T cells.
Keywords: CAR-T cells; delivery vector; immunotherapy; solid tumor.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Reshaping the tumor immune microenvironment to improve CAR-T cell-based cancer immunotherapy.Mol Cancer. 2024 Aug 26;23(1):175. doi: 10.1186/s12943-024-02079-8. Mol Cancer. 2024. PMID: 39187850 Free PMC article. Review.
-
Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells.Front Immunol. 2018 Jul 31;9:1717. doi: 10.3389/fimmu.2018.01717. eCollection 2018. Front Immunol. 2018. PMID: 30108584 Free PMC article. Review.
-
Engineering CAR-T Cells for Next-Generation Cancer Therapy.Cancer Cell. 2020 Oct 12;38(4):473-488. doi: 10.1016/j.ccell.2020.07.005. Epub 2020 Jul 30. Cancer Cell. 2020. PMID: 32735779 Review.
-
Prospects for chimeric antigen receptor-modified T cell therapy for solid tumors.Mol Cancer. 2018 Jan 12;17(1):7. doi: 10.1186/s12943-018-0759-3. Mol Cancer. 2018. PMID: 29329591 Free PMC article. Review.
-
Paving New Roads for CARs.Trends Cancer. 2019 Oct;5(10):583-592. doi: 10.1016/j.trecan.2019.09.005. Epub 2019 Oct 19. Trends Cancer. 2019. PMID: 31706506 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

