The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model
- PMID: 40244025
- PMCID: PMC11989926
- DOI: 10.3390/ijms26073222
The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model
Abstract
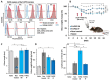
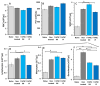
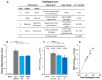
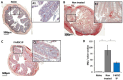
Inflammatory Bowel Disease (IBD) is a multifactorial gastrointestinal condition encompassing two major forms of intestinal inflammation: Crohn's disease (CD) and ulcerative colitis (UC). Both conditions are linked to auto-inflammatory reactions and genetic predispositions. Various drug therapies and biological treatments proposed to reduce IBD-associated inflammation. We induced IBD in a mouse model by stimulating bowel inflammation with an oral dextran sodium sulfate (DSS) beverage. Our novel cell therapy approach for IBD involves intramuscular (IM) and intraperitoneal (IP) delivery of non-matched, expanded, potent xenogeneic fetal human mesenchymal stromal cells (f-hPSCs) in 2 × 106 cell injections. This cell therapy has already been shown previously to induce pro-regenerative and anti-inflammatory effects in different systemic and local disorders, where the injected f-hPSCs were shown to respond to the stress of the host and secrete the adequate secretome in response to this stress. In the current study, the IP-injected f-hPSCs treatment of the DSS-induced IBD enhanced the regenerative processes of the damaged bowel and reduced the inflammatory process. This was associated with rapid regain of the mice's weight and a decrease in inflammation-associated parameters, such as colon edema, bowel shortening, and a threefold increase in bowel mass, as estimated by increased colon weight and reduced length. This ratio best emphasized the induced inflammatory response associated with the decrease in the inflamed colon length with an increase in its mass. Although IM f-hPSCs delivery was somehow effective by a few parameters, the IP delivery produced a superior response. The IP f-hPSCs treated mice lost only ~15% of their weight at the peak of the IBD effect, compared to ~25% in untreated mice. A reduction in the inflammatory response of the gut was also indicated by a decrease in neutrophil infiltration, as assayed by a myeloperoxidase (MPO) assay. Additionally, a significant improvement in the histological score of the gut and faster recovery to 90% of its original size was observed. These findings suggest that f-hPSC treatments could serve as an effective and safe anti-inflammatory and pro-regenerative treatment for IBD.
Keywords: C3H mouse model; adult cell therapy; cell therapy; expanded fetal human placental stromal cells (f-hPSCs); inflammatory bowel disease (IBD).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bandzar S., Gupta S., Platt M.O. Crohn’s disease: A review of treatment options and current research. Cell. Immunol. 2013;286:45–52. - PubMed
-
- Singh S., Andersen N.N., Andersson M., Loftus E.V., Jess T., Jr. Comparison of infliximab with adalimumab in 827 biologic-naive patients with Crohn’s disease: A population-based Danish cohort study. Aliment. Pharmacol. Ther. 2018;47:596–604. - PubMed
-
- Feagan B.G., Rutgeerts P., Sands B.E., Hanauer S., Colombel J.F., Sandborn W.J., Van Assche G., Axler J., Kim H.J., Danese S., et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013;369:699–710. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

