Growth Hormone-Releasing Hormone Antagonists Increase Radiosensitivity in Non-Small Cell Lung Cancer Cells
- PMID: 40244089
- PMCID: PMC11990011
- DOI: 10.3390/ijms26073267
Growth Hormone-Releasing Hormone Antagonists Increase Radiosensitivity in Non-Small Cell Lung Cancer Cells
Abstract
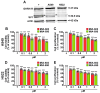
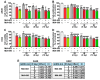
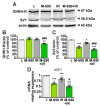
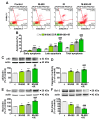
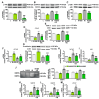
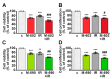
Growth hormone-releasing hormone (GHRH) antagonists exert antitumor functions in different experimental cancers. However, their role in combination with radiotherapy in non-small cell lung cancer (NSCLC) remains unknown. Therefore, we investigated the radiosensitizing effect of GHRH antagonists in NSCLC. A549 and H522 NSCLC cell lines were exposed to ionizing radiation (IR) and GHRH antagonists MIA-602 and MIA-690, either individually or in combination. Cell viability and proliferation were evaluated by MTT, BrdU, flow cytofluorimetry, and clonogenic assays; gene and protein expression, signaling pathways, and apoptosis were analyzed by real-time PCR, Western blot, annexin staining, and caspase-3 assay. GHRH antagonists showed antitumor effects alone and potentiated IR-induced inhibition of cell viability and proliferation. The combination of MIA-690 and IR decreased the expression of GHRH receptor, its oncogenic splice variant 1, and IGF1 mRNA levels. Additionally, cell cycle inhibitors and proapoptotic markers were upregulated, whereas cyclins, oncogenic MYC, and the antiapoptotic protein Bcl-2 were downregulated. Radioresistance was prevented by MIA-690, which also blunted epithelial-mesenchymal transition by enhancing E-cadherin and reducing mesenchymal, oxidative, and proangiogenic effectors. Finally, both MIA-602 and MIA-690 enhanced radiosensitivity in primary human NSCLC cells. These findings highlight the potential of GHRH antagonists as radiosensitizers in NSCLC treatment.
Keywords: GHRH; GHRH antagonists; NSCLC; ionizing radiation; radiosensitivity.
Conflict of interest statement
R.C., X.Z. and W.S. are co-inventors on the patents for GHRH analogs, assigned to the University of Miami and Veterans Affairs Medical Center. The remaining authors declare no conflicts of interest.
Figures

Similar articles
-
Inhibition of experimental small-cell and non-small-cell lung cancers by novel antagonists of growth hormone-releasing hormone.Int J Cancer. 2018 Jun 1;142(11):2394-2404. doi: 10.1002/ijc.31308. Epub 2018 Mar 1. Int J Cancer. 2018. PMID: 29435973
-
Novel GHRH antagonists suppress the growth of human malignant melanoma by restoring nuclear p27 function.Cell Cycle. 2014;13(17):2790-7. doi: 10.4161/15384101.2015.945879. Cell Cycle. 2014. PMID: 25486366 Free PMC article.
-
Mogrol-mediated enhancement of radiotherapy sensitivity in non-small cell lung cancer: a mechanistic study.Am J Physiol Cell Physiol. 2024 Jun 1;326(6):C1753-C1768. doi: 10.1152/ajpcell.00684.2023. Epub 2024 Apr 29. Am J Physiol Cell Physiol. 2024. PMID: 38682239
-
A novel approach for the treatment of AML, through GHRH antagonism: MIA-602.Rev Endocr Metab Disord. 2025 Jun;26(3):483-491. doi: 10.1007/s11154-024-09917-6. Epub 2024 Oct 17. Rev Endocr Metab Disord. 2025. PMID: 39417961 Free PMC article. Review.
-
The development of growth hormone-releasing hormone analogs: Therapeutic advances in cancer, regenerative medicine, and metabolic disorders.Rev Endocr Metab Disord. 2025 Jun;26(3):385-396. doi: 10.1007/s11154-024-09929-2. Epub 2024 Nov 26. Rev Endocr Metab Disord. 2025. PMID: 39592529 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

