Autophagy and Its Association with Macrophages in Clonal Hematopoiesis Leading to Atherosclerosis
- PMID: 40244103
- PMCID: PMC11989900
- DOI: 10.3390/ijms26073252
Autophagy and Its Association with Macrophages in Clonal Hematopoiesis Leading to Atherosclerosis
Abstract
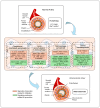
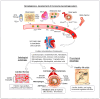
Atherosclerosis, a chronic inflammatory disease characterized by lipid accumulation and immune cell infiltration, is linked to plaque formation and cardiovascular events. While traditionally associated with lipid metabolism and endothelial dysfunction, recent research highlights the roles of autophagy and clonal hematopoiesis (CH) in its pathogenesis. Autophagy, a cellular process crucial for degrading damaged components, regulates macrophage homeostasis and inflammation, both of which are pivotal in atherosclerosis. In macrophages, autophagy influences lipid metabolism, cytokine regulation, and oxidative stress, helping to prevent plaque instability. Defective autophagy exacerbates inflammation, impairs cholesterol efflux, and accelerates disease progression. Additionally, autophagic processes in endothelial cells and smooth muscle cells further contribute to atherosclerotic pathology. Recent studies also emphasize the interplay between autophagy and CH, wherein somatic mutations in genes like TET2, JAK2, and DNMT3A drive immune cell expansion and enhance inflammatory responses in atherosclerotic plaques. These mutations modify macrophage function, intensifying the inflammatory environment and accelerating atherosclerosis. Chaperone-mediated autophagy (CMA), a selective form of autophagy, also plays a critical role in regulating macrophage inflammation by degrading pro-inflammatory cytokines and oxidized low-density lipoprotein (ox-LDL). Impaired CMA activity leads to the accumulation of these substrates, activating the NLRP3 inflammasome and worsening inflammation. Preclinical studies suggest that pharmacologically activating CMA may mitigate atherosclerosis progression. In animal models, reduced CMA activity accelerates plaque instability and increases inflammation. This review highlights the importance of autophagic regulation in macrophages, focusing on its role in inflammation, plaque formation, and the contributions of CH. Building upon current advances, we propose a hypothesis in which autophagy, programmed cell death, and clonal hematopoiesis form a critical intrinsic axis that modulates the fundamental functions of macrophages, playing a complex role in the development of atherosclerosis. Understanding these mechanisms offers potential therapeutic strategies targeting autophagy and inflammation to reduce the burden of atherosclerotic cardiovascular disease.
Keywords: atherosclerosis; chaperone-mediated autophagy (CMA); clonal hematopoiesis (CH); inflammatory; macrophage; programmed cell death.
Conflict of interest statement
The authors declare no conflicts of interest. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Challenges and advances in the management of inflammation in atherosclerosis.J Adv Res. 2025 May;71:317-335. doi: 10.1016/j.jare.2024.06.016. Epub 2024 Jun 22. J Adv Res. 2025. PMID: 38909884 Free PMC article. Review.
-
MicroRNA-761 modulates foam cell formation and inflammation through autophagy in the progression of atherosclerosis.Mol Cell Biochem. 2020 Nov;474(1-2):135-146. doi: 10.1007/s11010-020-03839-y. Epub 2020 Aug 8. Mol Cell Biochem. 2020. PMID: 32772311
-
Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE-/- mice.Oncotarget. 2016 Nov 22;7(47):76423-76436. doi: 10.18632/oncotarget.13121. Oncotarget. 2016. PMID: 27821816 Free PMC article.
-
Deficient Chaperone-Mediated Autophagy Promotes Inflammation and Atherosclerosis.Circ Res. 2021 Dec 3;129(12):1141-1157. doi: 10.1161/CIRCRESAHA.121.318908. Epub 2021 Oct 27. Circ Res. 2021. PMID: 34704457 Free PMC article.
-
LncRNA-modulated autophagy in plaque cells: a new paradigm of gene regulation in atherosclerosis?Aging (Albany NY). 2020 Nov 4;12(21):22335-22349. doi: 10.18632/aging.103786. Epub 2020 Nov 4. Aging (Albany NY). 2020. PMID: 33154191 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- 82200513/National Natural Science Foundation of China
- 202405/Sanqin Talent Innovation Team Program
- 2022ZDLSF05-15/Key Program of Shaanxi Provincial Science and Technology Department
- 2022ZDLSF04-01/Key Program of Shaanxi Provincial Science and Technology Department
- 20220317/haanxi Association for Science and Technology Youth Talent Support Program
- 959202313083/Young Talent Fund of Xi'an Association for Science and Technology
- 20JS138/Key Program of Shaanxi Provincial Education Department
- 121524083/S202411840083/College Student Innovation Training Program of Xi'an Medical University
- 205152353/208240383/Scientific and technological capabilities enhancement project from Xi'an Medical University
- L2024-ZDYF-ZDYF-SF-0054/Key Program of Xianyang Science and Technology Department
- RCYJ202401/Talent Program of Xi'an Medical University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

