Host Proteins in Echinococcus multilocularis Metacestodes
- PMID: 40244114
- PMCID: PMC11989879
- DOI: 10.3390/ijms26073266
Host Proteins in Echinococcus multilocularis Metacestodes
Abstract
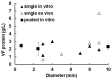
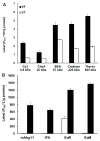
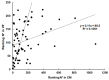
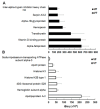
Metacestodes of Echinococcus multilocularis are the causative agents of alveolar echinococcosis, a neglected, life-threatening, zoonotic disease. To study these metacestodes in vitro, a model system using a culture medium conditioned by rat hepatoma cells is available. A key question is how the parasite interacts with the host and, in particular, which host-derived compounds are taken up. In this study, we focus on the uptake of host-derived proteins. Studies with artificially labeled proteins suggest that this uptake may occur independently of protein size or charge. Closer investigation using proteomics draws, however, a different picture. Of 1170 host (i.e., rat or bovine) proteins as identified by LC-MS/MS-based proteomics present in the culture medium, only 225 are found in metacestode vesicle tissue or fluid. Moreover, their relative abundances differ. Serum albumin, the most abundant culture medium host protein, is only the third most abundant protein in vesicle fluid, where Alpha-2-HS-glycoprotein becomes the most abundant protein. In vesicle fluid obtained ex vivo from experimentally infected mice, the situation is again different, with histone isoforms as the most abundant proteins. This suggests that while maintaining their internal milieu constant, metacestodes may adjust the spectrum of host proteins taken up. Potential uptake mechanisms and functions are discussed.
Keywords: helminth proteomics; homeostasis; host-parasite interaction; model system; systems biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Targeted and non-targeted proteomics to characterize the parasite proteins of Echinococcus multilocularis metacestodes.Front Cell Infect Microbiol. 2023 May 30;13:1170763. doi: 10.3389/fcimb.2023.1170763. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37325510 Free PMC article.
-
Extracellular non-coding RNA signatures of the metacestode stage of Echinococcus multilocularis.PLoS Negl Trop Dis. 2020 Nov 30;14(11):e0008890. doi: 10.1371/journal.pntd.0008890. eCollection 2020 Nov. PLoS Negl Trop Dis. 2020. PMID: 33253209 Free PMC article.
-
In vitro metabolomic footprint of the Echinococcus multilocularis metacestode.Sci Rep. 2019 Dec 19;9(1):19438. doi: 10.1038/s41598-019-56073-y. Sci Rep. 2019. PMID: 31857639 Free PMC article.
-
Echinococcus multilocularis: the parasite-host interplay.Exp Parasitol. 2008 Aug;119(4):447-452. doi: 10.1016/j.exppara.2008.03.002. Epub 2008 Mar 14. Exp Parasitol. 2008. PMID: 18410929 Review.
-
[Research advances in interplay of host immune mechanism and Echinococcus multilocularis metacestodes].Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2012 Oct 30;30(5):401-5. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2012. PMID: 23484285 Review. Chinese.
References
-
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F., Rokni M.B., Zhou X.N., Fevre E.M., Sripa B., et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12:e1001920. doi: 10.1371/journal.pmed.1001920. - DOI - PMC - PubMed
-
- Casulli A., Abela-Ridder B., Petrone D., Fabiani M., Bobic B., Carmena D., Soba B., Zerem E., Gargate M.J., Kuzmanovska G., et al. Unveiling the incidences and trends of the neglected zoonosis cystic echinococcosis in Europe: A systematic review from the MEmE project. Lancet Infect. Dis. 2023;23:e95–e107. doi: 10.1016/S1473-3099(22)00638-7. - DOI - PubMed
-
- Trotz-Williams L.A., Mercer N.J., Walters J.M., Wallace D., Gottstein B., Osterman-Lind E., Boggild A.K., Peregrine A.S. Public health follow-up of suspected exposure to Echinococcus multilocularis in Southwestern Ontario. Zoonoses Public Health. 2017;64:460–467. doi: 10.1111/zph.12326. - DOI - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

