O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies
- PMID: 40244145
- PMCID: PMC11989994
- DOI: 10.3390/ijms26073303
O-GlcNAcylation and Phosphorylation Crosstalk in Vascular Smooth Muscle Cells: Cellular and Therapeutic Significance in Cardiac and Vascular Pathologies
Abstract
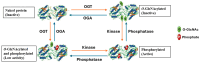
More than 400 different types of post-translational modifications (PTMs), including O-GlcNAcylation and phosphorylation, combine to co-ordinate almost all aspects of protein function. Often, these PTMs overlap and the specific relationship between O-GlcNAcylation and phosphorylation has drawn much attention. In the last decade, the significance of this dynamic crosstalk has been linked to several chronic pathologies of cardiovascular origin. However, very little is known about the pathophysiological significance of this crosstalk for vascular smooth muscle cell dysfunction in cardiovascular disease. O-GlcNAcylation occurs on serine and threonine residues which are also targets for phosphorylation. A growing body of research has now emerged linking altered vascular integrity and homeostasis with highly regulated crosstalk between these PTMs. Additionally, a significant body of evidence indicates that O-GlcNAcylation is an important contributor to the pathogenesis of neointimal hyperplasia and vascular restenosis responsible for long-term vein graft failure. In this review, we evaluate the significance of this dynamic crosstalk and its role in cardiovascular pathologies, and the prospects of identifying possible targets for more effective therapeutic interventions.
Keywords: O-GlcNAcylation; crosstalk; phosphorylation; vascular smooth muscle cells.
Conflict of interest statement
No conflicts of interest are declared by the authors.
Figures

Similar articles
-
O-Linked β-N-Acetylglucosamine Modification of A20 Enhances the Inhibition of NF-κB (Nuclear Factor-κB) Activation and Elicits Vascular Protection After Acute Endoluminal Arterial Injury.Arterioscler Thromb Vasc Biol. 2018 Jun;38(6):1309-1320. doi: 10.1161/ATVBAHA.117.310468. Epub 2018 Apr 5. Arterioscler Thromb Vasc Biol. 2018. PMID: 29622561
-
Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus.Circ Res. 2014 Mar 28;114(7):1094-102. doi: 10.1161/CIRCRESAHA.114.302968. Epub 2014 Feb 13. Circ Res. 2014. PMID: 24526702 Free PMC article.
-
Crosstalk between phosphorylation and O-GlcNAcylation: friend or foe.FEBS J. 2018 Sep;285(17):3152-3167. doi: 10.1111/febs.14491. Epub 2018 May 13. FEBS J. 2018. PMID: 29717537 Review.
-
Revascularisation of type 2 diabetics with coronary artery disease: Insights and therapeutic targeting of O-GlcNAcylation.Nutr Metab Cardiovasc Dis. 2021 May 6;31(5):1349-1356. doi: 10.1016/j.numecd.2021.01.017. Epub 2021 Feb 2. Nutr Metab Cardiovasc Dis. 2021. PMID: 33812732 Review.
-
Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse.Mol Cell Proteomics. 2012 Aug;11(8):215-29. doi: 10.1074/mcp.O112.018366. Epub 2012 May 29. Mol Cell Proteomics. 2012. PMID: 22645316 Free PMC article.
Cited by
-
Reactive Oxygen Species: A Double-Edged Sword in the Modulation of Cancer Signaling Pathway Dynamics.Cells. 2025 Aug 6;14(15):1207. doi: 10.3390/cells14151207. Cells. 2025. PMID: 40801639 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

