Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury
- PMID: 40244153
- PMCID: PMC11989546
- DOI: 10.3390/ijms26073285
Depletion of Cell Adhesion Molecule L1 from Microglia and Macrophages Reduces Recovery After Spinal Cord Injury
Abstract
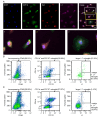
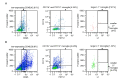
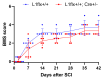
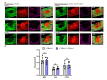
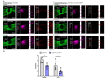
The young mammalian central nervous system regenerates after spinal cord injury and recovers locomotion, whereas adult mice only show limited recovery that depends on the injury severity, genetic background, and physical therapy. At the molecular level, key regulators that contribute to recovery are cell adhesion molecules, such as L1CAM (L1). At the cell surface, L1 functions as a homotypic receptor that signal-transduces crucial functions in neuronal migration and survival, neurite outgrowth, myelination, formation of synapses, and synaptic plasticity. In the adult central nervous system, L1 is expressed only by neurons. We now show that L1 is unexpectedly also expressed by 26% microglia, freshly isolated from a 7-day-old mouse brain. At postnatal day 21, only 3% of microglia are L1-positive. Using a mouse mutant in which L1 is deleted specifically in monocytes of 10- to 14-week-old mice, functional recovery was reduced up to 4 weeks after injury at lower thoracic spinal levels. Also, NF200-immunoreactive and 5-HT-immunoreactive fibers were found decreased below the injury site as compared to wild-type mice. In conclusion, microglial cells that express L1 stimulate neurite outgrowth in vitro, improve functional recovery after spinal cord injury in adult mice, and increase fiber densities caudal to the lesion site.
Keywords: Basso mouse scale; L1CAM; functional recovery; microglia; spinal cord injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Xiao Z., Tang F., Tang J., Yang H., Zhao Y., Chen B., Han S., Wang N., Li X., Cheng S., et al. One-Year Clinical Study of NeuroRegen Scaffold Implantation Following Scar Resection in Complete Chronic Spinal Cord Injury Patients. Sci. China Life Sci. 2016;59:647–655. doi: 10.1007/s11427-016-5080-z. - DOI - PubMed
-
- Zhu H., Poon W., Liu Y., Leung G.K.-K., Wong Y., Feng Y., Ng S.C.P., Tsang K.S., Sun D.T.F., Yeung D.K., et al. Phase I–II Clinical Trial Assessing Safety and Efficacy of Umbilical Cord Blood Mononuclear Cell Transplant Therapy of Chronic Complete Spinal Cord Injury. Cell Transplant. 2016;25:1925–1943. doi: 10.3727/096368916X691411. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

