Parkinson's Disease: The Neurodegenerative Enigma Under the "Undercurrent" of Endoplasmic Reticulum Stress
- PMID: 40244210
- PMCID: PMC11989508
- DOI: 10.3390/ijms26073367
Parkinson's Disease: The Neurodegenerative Enigma Under the "Undercurrent" of Endoplasmic Reticulum Stress
Abstract
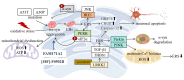
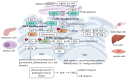
Parkinson's disease (PD), a prevalent neurodegenerative disorder, demonstrates the critical involvement of endoplasmic reticulum stress (ERS) in its pathogenesis. This review comprehensively examines the role and molecular mechanisms of ERS in PD. ERS represents a cellular stress response triggered by imbalances in endoplasmic reticulum (ER) homeostasis, induced by factors such as hypoxia and misfolded protein aggregation, which activate the unfolded protein response (UPR) through the inositol-requiring enzyme 1 (IRE1), protein kinase R-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6) pathways. Clinical, animal model, and cellular studies have consistently demonstrated a strong association between PD and ERS. Abnormal expression of ERS-related molecules in PD patients' brains and cerebrospinal fluid (CSF) correlates with disease progression. In animal models (e.g., Drosophila and mice), ERS inhibition alleviates dopaminergic neuronal damage. Cellular experiments reveal that PD-mimicking pathological conditions induce ERS, while interactions between ERS and mitochondrial dysfunction promote neuronal apoptosis. Mechanistically, (1) pathological aggregation of α-synuclein (α-syn) and ERS mutually reinforce dopaminergic neuron damage; (2) leucine-rich repeat kinase 2 (LRRK2) gene mutations induce ERS through thrombospondin-1 (THBS1)/transforming growth factor beta 1 (TGF-β1) interactions; (3) molecules such as Parkin and PTEN-induced kinase 1 (PINK1) regulate ERS in PD. Furthermore, ERS interacts with mitochondrial dysfunction, oxidative stress, and neuroinflammation to exacerbate neuronal injury. Emerging therapeutic strategies show significant potential, including artificial intelligence (AI)-assisted drug design targeting ERS pathways and precision medicine approaches exploring non-pharmacological interventions such as personalized electroacupuncture. Future research should focus on elucidating ERS-related mechanisms and identifying novel therapeutic targets to develop more effective treatments for PD patients, ultimately improving their quality of life.
Keywords: Parkinson’s disease; endoplasmic reticulum stress; molecular mechanisms; therapeutic strategies; unfolded protein response; α-synuclein.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
H50Q mutation in alpha-Synuclein impairs the insulin signaling pathway and induces neuroinflammation in the Drosophila model.Exp Cell Res. 2025 Apr 1;447(1):114460. doi: 10.1016/j.yexcr.2025.114460. Epub 2025 Feb 20. Exp Cell Res. 2025. PMID: 39986600
-
Endoplasmic Reticulum Stress Inhibition Promotes Mitophagy via Miro1 Reduction to Rescue Mitochondrial Dysfunction and Protect Dopamine Neurons in Parkinson's Disease.Cell Mol Neurobiol. 2025 May 29;45(1):53. doi: 10.1007/s10571-025-01575-9. Cell Mol Neurobiol. 2025. PMID: 40439946 Free PMC article.
-
IRE1 promotes neurodegeneration through autophagy-dependent neuron death in the Drosophila model of Parkinson's disease.Cell Death Dis. 2019 Oct 22;10(11):800. doi: 10.1038/s41419-019-2039-6. Cell Death Dis. 2019. PMID: 31641108 Free PMC article.
-
ER stress and Parkinson's disease: Pathological inputs that converge into the secretory pathway.Brain Res. 2016 Oct 1;1648(Pt B):626-632. doi: 10.1016/j.brainres.2016.04.042. Epub 2016 Apr 19. Brain Res. 2016. PMID: 27103567 Review.
-
The role of endoplasmic reticulum stress in neurodegenerative disease.Apoptosis. 2017 Jan;22(1):1-26. doi: 10.1007/s10495-016-1296-4. Apoptosis. 2017. PMID: 27815720 Review.
Cited by
-
Actual Data on Essential Trace Elements in Parkinson's Disease.Nutrients. 2025 May 29;17(11):1852. doi: 10.3390/nu17111852. Nutrients. 2025. PMID: 40507121 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

