Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice
- PMID: 40244217
- PMCID: PMC11989601
- DOI: 10.3390/ijms26073325
Repeated Administrations of Polyphenolic Extracts Prevent Chronic Reflexive and Non-Reflexive Neuropathic Pain Responses by Modulating Gliosis and CCL2-CCR2/CX3CL1-CX3CR1 Signaling in Spinal Cord-Injured Female Mice
Abstract
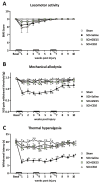
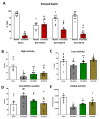
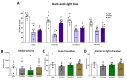
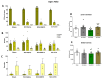
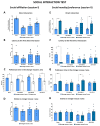
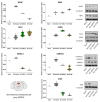
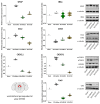
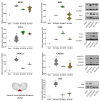
Neuropathic pain after spinal cord injury lacks any effective treatments, often leading to chronic pain. This study tested whether the daily administration of fully characterized polyphenolic extracts from grape stalks and coffee could prevent both reflexive and non-reflexive chronic neuropathic pain in spinal cord-injured mice by modulating the neuroimmune axis. Female CD1 mice underwent mild spinal cord contusion and received intraperitoneal extracts in weeks one, three, and six post-surgery. Reflexive pain responses were assessed weekly for up to 10 weeks, and non-reflexive pain was evaluated at the study's end. Neuroimmune crosstalk was investigated, focusing on glial activation and the expression of CCL2/CCR2 and CX3CL1/CX3CR1 in supraspinal pain-related areas, including the periaqueductal gray, rostral ventromedial medulla, anterior cingulate cortex, and amygdala. Repeated treatments prevented mechanical allodynia and thermal hyperalgesia, and also modulated non-reflexive pain. Moreover, they reduced supraspinal gliosis and regulated CCL2/CCR2 and CX3CL1/CX3CR1 signaling. Overall, the combination of polyphenols in these extracts may offer a promising pharmacological strategy to prevent chronic reflexive and non-reflexive pain responses by modifying central sensitization markers, not only at the contusion site but also in key supraspinal regions implicated in neuropathic pain. Overall, these data highlight the potential of polyphenolic extracts for spinal cord injury-induced chronic neuropathic pain.
Keywords: chemokines; chronic neuropathic pain; glia; neuroinflammation; polyphenols; spinal cord injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Polyphenolic grape stalk and coffee extracts attenuate spinal cord injury-induced neuropathic pain development in ICR-CD1 female mice.Sci Rep. 2022 Sep 2;12(1):14980. doi: 10.1038/s41598-022-19109-4. Sci Rep. 2022. PMID: 36056079 Free PMC article.
-
Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation.J Pain. 2014 May;15(5):516-26. doi: 10.1016/j.jpain.2014.01.492. Epub 2014 Jan 23. J Pain. 2014. PMID: 24462503
-
Transient Reflexive Pain Responses and Chronic Affective Nonreflexive Pain Responses Associated with Neuroinflammation Processes in Both Spinal and Supraspinal Structures in Spinal Cord-Injured Female Mice.Int J Mol Sci. 2023 Jan 16;24(2):1761. doi: 10.3390/ijms24021761. Int J Mol Sci. 2023. PMID: 36675275 Free PMC article.
-
Chemokines, neuronal-glial interactions, and central processing of neuropathic pain.Pharmacol Ther. 2010 Apr;126(1):56-68. doi: 10.1016/j.pharmthera.2010.01.002. Epub 2010 Feb 1. Pharmacol Ther. 2010. PMID: 20117131 Free PMC article. Review.
-
Analysis of the Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Traumatic Brain and Spinal Cord Injury: Insight into Recent Advances in Actions of Neurochemokine Agents.Mol Neurobiol. 2017 Apr;54(3):2167-2188. doi: 10.1007/s12035-016-9787-4. Epub 2016 Mar 1. Mol Neurobiol. 2017. PMID: 26927660 Free PMC article. Review.
References
-
- Warner F.M., Cragg J.J., Jutzeler C.R., Finnerup N.B., Werhagen L., Weidner N., Maier D., Kalke Y.B., Curt A., Kramer J.L.K. Progression of Neuropathic Pain after Acute Spinal Cord Injury: A Meta-Analysis and Framework for Clinical Trials. J. Neurotrauma. 2018;36:1461–1468. doi: 10.1089/NEU.2018.5960. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

