Parkinson's Spectrum Mechanisms in Pregnancy: Exploring Hypothetical Scenarios for MSA in the Era of ART
- PMID: 40244235
- PMCID: PMC11989403
- DOI: 10.3390/ijms26073348
Parkinson's Spectrum Mechanisms in Pregnancy: Exploring Hypothetical Scenarios for MSA in the Era of ART
Abstract
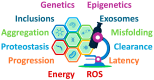
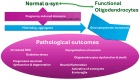
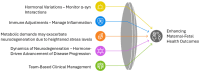
Multiple System Atrophy (MSA) is a rare, rapidly progressive neurodegenerative disorder marked by autonomic dysfunction, parkinsonism, and cerebellar ataxia. While predominantly affecting individuals in their fifth or sixth decade, advancements in assisted reproductive technologies (ART) have created new clinical scenarios involving pregnancies in women within MSA's typical onset range. Given the scarcity of documented MSA pregnancies, this review leverages insights from related Parkinson's spectrum mechanisms to explore hypothetical scenarios for how pregnancy-induced physiological changes might influence MSA progression. Pregnancy-induced hormonal fluctuations, including elevated estrogen and progesterone levels, may modulate α-synuclein aggregation and neuroinflammatory pathways. Immune adaptations, such as fetal microchimerism and Th2-biased immune profiles, introduce additional complexities, particularly in donor embryo pregnancies involving complex microchimerism. Metabolic demands and oxidative stress further intersect with these mechanisms, potentially accelerating disease progression. We analyze existing literature and theoretical models, emphasizing the need for interdisciplinary research. Clinical implications are discussed to propose evidence-based strategies for optimizing maternal-fetal outcomes. This paper identifies critical knowledge gaps and proposes avenues for future investigation to optimize maternal-fetal outcomes in this unique and underexplored clinical intersection.
Keywords: Parkinson’s spectrum; assisted reproductive technologies; epigenetics; hormonal changes; microchimerism; multiple system atrophy; pregnancy; α-synuclein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson's disease and progressive supranuclear palsy: a comparative investigation.Brain. 2010 Jan;133(Pt 1):172-88. doi: 10.1093/brain/awp282. Epub 2009 Nov 10. Brain. 2010. PMID: 19903734
-
Altered α-synuclein, parkin, and synphilin isoform levels in multiple system atrophy brains.J Neurochem. 2016 Jan;136(1):172-85. doi: 10.1111/jnc.13392. Epub 2015 Nov 11. J Neurochem. 2016. PMID: 26465922
-
Lysosomal response in relation to α-synuclein pathology differs between Parkinson's disease and multiple system atrophy.Neurobiol Dis. 2018 Jun;114:140-152. doi: 10.1016/j.nbd.2018.02.019. Epub 2018 Mar 2. Neurobiol Dis. 2018. PMID: 29505813
-
Models of multiple system atrophy.Exp Mol Med. 2019 Nov 18;51(11):1-10. doi: 10.1038/s12276-019-0346-8. Exp Mol Med. 2019. PMID: 31740682 Free PMC article. Review.
-
Animal modeling an oligodendrogliopathy--multiple system atrophy.Acta Neuropathol Commun. 2016 Feb 9;4:12. doi: 10.1186/s40478-016-0279-6. Acta Neuropathol Commun. 2016. PMID: 26860328 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

