Basic Pathological Mechanisms in Peripheral Nerve Diseases
- PMID: 40244242
- PMCID: PMC11989557
- DOI: 10.3390/ijms26073377
Basic Pathological Mechanisms in Peripheral Nerve Diseases
Abstract
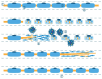
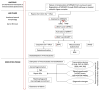
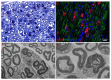
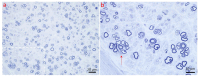
Pathological changes and the cellular and molecular mechanisms underlying axonopathy and myelinopathy are key to understanding a wide range of inherited and acquired peripheral nerve disorders. While the clinical indications for nerve biopsy have diminished over time, its diagnostic value remains significant in select conditions, offering a unique window into the pathophysiological processes of peripheral neuropathies. Evidence highlights the symbiotic relationship between axons and myelinating Schwann cells, wherein disruptions in axo-glial interactions contribute to neuropathogenesis. This review synthesizes recent insights into the pathological and molecular underpinnings of axonopathy and myelinopathy. Axonopathy encompasses Wallerian degeneration, axonal atrophy, and dystrophy. Although extensively studied in traumatic nerve injury, the mechanisms of axonal degeneration and Schwann cell-mediated repair are increasingly recognized as pivotal in non-traumatic disorders, including dying-back neuropathies. We briefly outline key transcription factors, signaling pathways, and epigenetic changes driving axonal regeneration. For myelinopathy, we discuss primary segmental demyelination and dysmyelination, characterized by defective myelin development. We describe paranodal demyelination in light of recent findings in nodopathies, emphasizing that it is not an exclusive indicator of demyelinating disorders. This comprehensive review provides a framework to enhance our understanding of peripheral nerve pathology and its implications for developing targeted therapies.
Keywords: axon; myelin; nerve; neuropathies; peripheral nervous system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


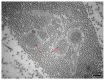






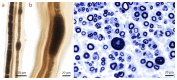
References
-
- Prada V., Massucco S., Venturi C., Geroldi A., Bellone E., Mandich P., Minuto M., Varaldo E., Mancardi G., Grandis M., et al. Diagnostic Value of Sural Nerve Biopsy: Retrospective Analysis of Clinical Cases from 1981 to 2017. Front. Neurol. 2019;10:1218. doi: 10.3389/fneur.2019.01218. - DOI - PMC - PubMed
-
- Sommer C.L., Brandner S., Dyck P.J., Harati Y., Lacroix C., Lammens M., Magy L., Mellgren S.I., Morbin M., Navarro C., et al. Peripheral Nerve Society Guideline on Processing and Evaluation of Nerve Biopsies. J. Peripher. Nerv. Syst. 2010;15:164–175. doi: 10.1111/j.1529-8027.2010.00276.x. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

