The Role of MSI Testing Methodology and Its Heterogeneity in Predicting Colorectal Cancer Immunotherapy Response
- PMID: 40244273
- PMCID: PMC11989282
- DOI: 10.3390/ijms26073420
The Role of MSI Testing Methodology and Its Heterogeneity in Predicting Colorectal Cancer Immunotherapy Response
Abstract
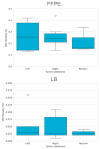
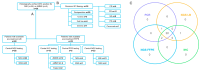
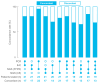
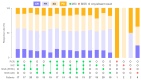
MSI is a crucial biomarker for selecting CRC patients for immunotherapy. Here, we analyze the first results from the observational prospective trial BLOOMSI (NCT06414304), which investigated the impact of MSI/dMMR testing methods and baseline tumor heterogeneity on treatment outcomes. Thirty MSI/dMMR+ CRC patients, who were candidates for immunotherapy, were enrolled. Depending on the local test used for MSI/dMMR, central PCR/IHC was performed. Baseline FFPE and liquid biopsy (LB) were analyzed with NGS. ORR (objective response rate) in the ITT population was 50% (95% CI, 31.3-68.7%). Concordance between local/central dMMR/MSI testing was 81%, and the concordance of IHC, PCR, NGS/FFPE, and NGS/LB was 68.4%. The ORR was similar for IHC+, PCR+, NGS/FFPE+, and NGS/LB+ patients (55.6%, 55.6%, 55%, and 57.9%, respectively). The ORR among patients with discordant IHC/PCR results was 0%, and the ORR among patients with NGS/LB-ORR was 25% (2/8 CR). Next, we performed quantitative MSI analysis, reflecting the clonality of MSI+ tumor cells. Multivariate analysis identified MSI clonality in FFPE (HR 0.63, 95% CI, 0.39-0.99, p = 0.0487) and LB (HR 3.05, 95% CI, 2.01-4.65, p < 0.00001) as independent predictors of progression. The ORR in patients with high clonality (≥7%, n = 4, NGS/LB) was 25%. We describe baseline methodological predictors of non-response to immunotherapy and propose a strategy for selecting potential non-responders. These findings warrant further investigation.
Keywords: MSI; colorectal cancer; dMMR; immunotherapy; liquid biopsy; next-generation sequencing.
Conflict of interest statement
Alexandra Lebedeva, Anastasiia Taraskina, Tatiana Grigoreva, Ekaterina Belova, Olesya Kuznetsova, Alexandra Kavun, Saida Aliyarova, Vladislav Mileyko, and Maxim Ivanov are currently employed by OncoAtlas LLC. Egor Veselovsky ended employment at OncoAtlas LLC in the past 24 months. The other co-authors have no conflicts of interest to disclose.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

