Mouse Models of HIV-Associated Atherosclerosis
- PMID: 40244289
- PMCID: PMC11989901
- DOI: 10.3390/ijms26073417
Mouse Models of HIV-Associated Atherosclerosis
Abstract
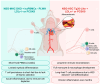
Cardiovascular disease (CVD) remains the leading cause of death worldwide. Several factors are implicated in the pathogenesis of CVD, and efforts have been made to reduce traditional risks, yet CVD remains a complex burden. Notably, people living with HIV (PLWH) are twice as likely to develop CVD compared to persons without HIV (PWoH). Intensive statin therapy, the first-line treatment to prevent cardiovascular events, is effective at reducing morbidity and mortality. However, statin therapy has not reduced the overall prevalence of CVD. Despite antiretroviral therapy (ART), and new guidelines for statin use, PLWH have persistent elevation of inflammatory markers, which is suggested to be a bigger driver of future cardiovascular events than low-density lipoprotein. Herein, we have summarized the development of atherosclerosis and highlighted mouse models of atherosclerosis in the presence and absence of HIV. Since most mouse strains have several mechanisms that are atheroprotective, researchers have developed mouse models to study CVD using dietary and genetic manipulations. In evaluating the current methodologies for studying HIV-associated atherosclerosis, we have detailed the benefits of integrating multi-omics analyses, genetic manipulations, and immune cell profiling within mouse models. These advanced approaches significantly enhance our capacity to address critical gaps in understanding the immune mechanisms driving CVD, including in the context of HIV.
Keywords: HIV; atherosclerosis; cardiovascular disease; inflammation; mouse models.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

