Biochemical Mechanism of Thai Fermented Soybean Extract on UVB-Induced Skin Keratinocyte Damage and Inflammation
- PMID: 40244292
- PMCID: PMC11989635
- DOI: 10.3390/ijms26073418
Biochemical Mechanism of Thai Fermented Soybean Extract on UVB-Induced Skin Keratinocyte Damage and Inflammation
Abstract
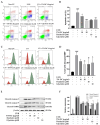
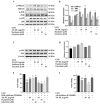
Ultraviolet B (UVB) radiation is a key factor contributing to photodamage in epidermal cells. This study investigated the protective effects of Thua Nao, a Thai fermented soybean product, against UVB-induced damage in human epidermal keratinocytes (HaCaT) and the underlying mechanisms. Thua Nao extract fractions were prepared using a solvent partition method. We found that the dichloromethane fraction (TN-DC), along with its isoflavones daidzein and glycitein, significantly protected against UVB-induced HaCaT cell death. This protection involved inhibiting caspase-9 and caspase-3 activation, thus preventing apoptosis. Additionally, treatment with TN-DC, daidzein, and glycitein suppressed the UVB-induced production of inflammatory mediators, including interleukin-6 (IL-6), IL-8, inducible nitric oxide synthase, and cyclooxygenase-2. These protective effects were associated with reduced intracellular reactive oxygen species and enhanced the levels of antioxidant enzymes, including superoxide dismutase and glutathione peroxidase 4. Signaling pathway analysis revealed that TN-DC activated the pro-survival ERK1/2 and Akt pathways while decreased the phosphorylation of JNK in UVB-exposed cells. On the other hand, daidzein and glycitein enhanced ERK1/2 activation and reduced the phosphorylation of JNK and p38 MAPKs. The involvement of ERK1/2 and Akt activation in cell survival was confirmed using specific inhibitors. Thus, TN-DC and its isoflavones protects keratinocytes from UVB-induced oxidative damage and inflammation by modulating MAPKs and Akt signaling.
Keywords: HaCaT keratinocyte; UVB; anti-apoptosis; fermented soybean; skin damage.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

