Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2- Breast Cancer
- PMID: 40244377
- PMCID: PMC11989623
- DOI: 10.3390/ijms26073438
Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2- Breast Cancer
Abstract
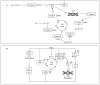
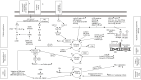
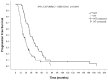
Approximately 70-80% of breast cancers are estrogen receptor-positive (ER+), with 65% of these cases also being progesterone receptor-positive (ER+PR+). In most cases of ER+ advanced breast cancer, endocrine therapy (ET) serves as the first-line treatment, utilizing various drugs that inhibit ER signaling. These include tamoxifen, a selective estrogen receptor modulator (SERM); fulvestrant, a selective estrogen receptor degrader (SERD); and aromatase inhibitors (AIs), which block estrogen synthesis. However, intrinsic or acquired hormone resistance eventually develops, leading to disease progression. The combination of ET with cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6is) has been shown to significantly increase progression-free survival (PFS) and, in some cases, overall survival (OS). CDK4/6is works by arresting the cell cycle in the G1 phase, preventing DNA synthesis, and enhancing the efficacy of ET. This review highlights the key mechanisms of resistance to ET, whether used alone or in combination with biological agents, as well as emerging therapeutic strategies aimed at overcoming resistance. Addressing ET resistance remains a work in progress, and in the near future, better patient selection for different therapeutic approaches is expected through the identification of more precise biological and genetic markers. In particular, liquid biopsy may provide a real-time portrait of the disease, offering insights into mechanisms driving ET resistance and cancer progression.
Keywords: CDK4/6 inhibitors; advanced ER+/HER2− breast cancer; endocrine resistance; endocrine therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
EGFR and HER2 hyper-activation mediates resistance to endocrine therapy and CDK4/6 inhibitors in ER+ breast cancer.Cancer Lett. 2024 Jul 1;593:216968. doi: 10.1016/j.canlet.2024.216968. Epub 2024 May 23. Cancer Lett. 2024. PMID: 38788968
-
Resistance to cyclin-dependent kinase (CDK) 4/6 inhibitors confers cross-resistance to other CDK inhibitors but not to chemotherapeutic agents in breast cancer cells.Breast Cancer. 2021 Jan;28(1):206-215. doi: 10.1007/s12282-020-01150-8. Epub 2020 Aug 28. Breast Cancer. 2021. PMID: 32860163 Free PMC article.
-
Gene expression analysis in circulating tumour cells to determine resistance to CDK4/6 inhibitors plus endocrine therapy in HR + /HER2- metastatic breast cancer patients.J Transl Med. 2025 Apr 4;23(1):400. doi: 10.1186/s12967-025-06374-w. J Transl Med. 2025. PMID: 40186268 Free PMC article.
-
Post-progression treatment options after CDK4/6 inhibitors in hormone receptor-positive, HER2-negative metastatic breast cancer.Cancer Treat Rev. 2025 Apr;135:102924. doi: 10.1016/j.ctrv.2025.102924. Epub 2025 Mar 20. Cancer Treat Rev. 2025. PMID: 40121890 Review.
-
Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.Breast Cancer Res Treat. 2020 Feb;180(1):21-32. doi: 10.1007/s10549-020-05528-2. Epub 2020 Jan 22. Breast Cancer Res Treat. 2020. PMID: 31970560 Review.
References
-
- Allison K.H., Hammond M.E.H., Dowsett M., McKernin S.E., Carey L.A., Fitzgibbons P.L., Hayes D.F., Lakhani S.R., Chavez-MacGregor M., Perlmutter J., et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. - DOI - PubMed
-
- NCCN Guidelines, Breast Cancer. [(accessed on 15 March 2025)]. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/....
-
- ESMO Clinical Practice Guidelines: Breast Cancer. [(accessed on 15 March 2025)]. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practi....
-
- Ferro A., Campora M., Caldara A., De Lisi D., Lorenzi M., Monteverdi S., Mihai R., Bisio A., Dipasquale M., Caffo O., et al. Novel Treatment Strategies for Hormone Receptor (HR)-Positive, HER2-Negative Metastatic Breast Cancer. J. Clin. Med. 2024;13:3611. doi: 10.3390/jcm13123611. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

