Glycodeoxycholic acid alleviates central precocious puberty by modulating gut microbiota and metabolites in high-fat diet-fed female rats
- PMID: 40244411
- PMCID: PMC12006580
- DOI: 10.1007/s00018-025-05680-2
Glycodeoxycholic acid alleviates central precocious puberty by modulating gut microbiota and metabolites in high-fat diet-fed female rats
Abstract
Objective: Central precocious puberty (CPP) is a common pediatric endocrine disorder and a significant global public health concern. Emerging evidence suggests an association between bile acids (BAs) and CPP, although their regulatory roles and underlying mechanisms remain poorly understood.
Methods: We conducted untargeted metabolomics and targeted BA analysis on serum samples from female rats with high-fat diet-induced CPP to identify metabolites potentially involved in regulating puberty through modulation of Sirt1 and Kiss1 expression in the hypothalamus. Identified BAs were then administered via gavage to female rats with CPP to assess their effects. To explore the mechanisms by which these BAs affect the development of CPP, gut microbiota and their metabolites were analyzed using 16S rRNA sequencing and untargeted metabolomics.
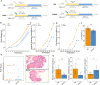
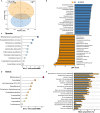
Results: Our findings revealed significant reductions in glycodeoxycholic acid (GDCA) and glycoursodeoxycholic acid (GUDCA) levels in female rats with CPP. GDCA treatment delayed the onset of puberty, accompanied by alterations in the gut microbiota functions and metabolic pathways related to oxidative stress (OS) and fatty acid metabolism. Mediation analysis suggested that OS-related metabolites, including gamma-glutamylcysteine and malonic acid, which increased with the abundance of Lachnospiraceae UCG-001, facilitated the reduction of Sirt1 expression. Additionally, pregnenolone appeared to suppress the beneficial effect of Parasutterella in enhancing Sirt1 expression.
Conclusion: This study demonstrates that GDCA exhibits a potential therapeutic effect on CPP through a unique mechanism that involves gut microbiota modulation, alterations in serum metabolites, and changes in the expression of key regulatory factors Sirt1.
Keywords: Sirt1 regulation; Central precocious puberty; Glycodeoxycholic acid; Gut microbiota; Gut-brain axis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. The animal study was approved by the Ethics Committee of Public Health at Shandong University with the permission number LL202303027. Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- Latronico AC, Brito VN, Carel J-CJTD (2016) endocrinology. Causes, diagnosis, and treatment of central precocious puberty. 4(3):265–274 - PubMed
MeSH terms
Substances
Grants and funding
- 82370785/National Natural Science Foundation of China
- 82172320/National Natural Science Foundation of China
- ZR2024MH220/Shandong Provincial Natural Science Foundation
- Shandong University Outstanding Young Scholars Program/Shandong University Outstanding Young Scholars Program
- 2022FYH008/National Center for Women and Children's Health, China CDC "Maternal and Infants Nutrition and Health Research Programs"
LinkOut - more resources
Full Text Sources

