Clinical and dynamic circulating cytokines profile features of long-term progression-free survival benefit to immune checkpoint inhibitors in advanced non-small cell lung cancer
- PMID: 40244472
- PMCID: PMC12006652
- DOI: 10.1007/s00262-025-03984-7
Clinical and dynamic circulating cytokines profile features of long-term progression-free survival benefit to immune checkpoint inhibitors in advanced non-small cell lung cancer
Abstract
Background: Immune checkpoint inhibitors (ICIs) offer durable progression-free survival (PFS) benefit in a subset of patients with advanced non-small cell lung cancer (NSCLC). However, the predictors of long-term PFS (LTPFS) remain unclear.
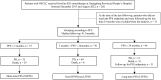
Methods: Advanced NSCLC patients receiving first-line ICIs monotherapy at Guangdong Lung Cancer Institute between December 2017 and August 2022 were identified. Predictive value of different characteristics was evaluated in LTPFS (PFS ≥ 24 months) compared with short-term PFS (STPFS, PFS ≤ 3 months). Circulating cytokine levels were evaluated in paired peripheral blood samples collected before and after ICIs treatment.
Results: Among 202 patients identified and 171 included (median follow-up: 41.0 months), 44 (25.7%) experienced LTPFS, associated with a 5-year overall survival (OS) rate of 81.2%. Squamous NSCLC, intermediate or poor lung immune prognostic index (LIPI) score, and liver metastases, were negatively associated with LTPFS. High tumor mutational burden (TMB, ≥ 10 mutations/megabase) was enriched in LTPFS compared to STPFS (P = 0.002). Patients with both high TMB and PD-L1 demonstrated the greatest survival benefit from first-line ICIs monotherapy (median PFS: 24.5 months, median OS: 67.0 months). Thirty-eight peripheral blood samples were collected before and after ICIs treatment from 10 patients with LTPFS and 9 with STPFS, which revealed increased CCL11 (P = 0.013) and decreased IL1RA (P = 0.001) and IL17A (P = 0.003) levels in LTPFS after ICIs treatment.
Conclusion: Distinct clinical characteristics, including TMB, PD-L1, pathologic subtypes, LIPI score, number of organs involved, metastatic sites, and dynamic circulating cytokines profile features, can distinguish NSCLC patients achieving LTPFS from those with STPFS following first-line ICIs monotherapy.
Keywords: Cytokine; Immunotherapy; Long-term; Non-small cell lung cancer; Predictive; Survival.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: Prof. Q. Zhou reports honoraria from AstraZeneca, Boehringer Ingelheim, BMS, Eli Lilly, MSD, Pfizer, Roche, and Sanofi outside the submitted work. The other authors have no competing interests to declare. Ethics approval statement and patient consent statement: The study was approved by the Ethics and Scientific Committees of Guangdong Provincial People’s Hospital [approval number: GDREC2019304H(R1)] and all the patients provided informed consent.
Figures




References
-
- de Castro G, Jr., Kudaba I, Wu YL, et al (2023) Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score >/= 1% in the KEYNOTE-042 study. J Clin Oncol 41:1986–1991. 10.1200/JCO.21.02885 - DOI - PMC - PubMed
-
- Perol M, Felip E, Dafni U et al (2022) Effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol 33:511–521. 10.1016/j.annonc.2022.02.008 - DOI - PubMed
-
- Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830. 10.1016/S0140-6736(18)32409-7 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

