Prevention of bicalutamide-induced breast events in patients with prostate cancer: a meta-analysis of randomized controlled trials
- PMID: 40244528
- PMCID: PMC12313775
- DOI: 10.1007/s40618-025-02583-8
Prevention of bicalutamide-induced breast events in patients with prostate cancer: a meta-analysis of randomized controlled trials
Abstract
Purpose: This study aimed to quantitatively assess the effectiveness of tamoxifen, anastrozole, and radiotherapy in preventing bicalutamide-induced breast events-specifically gynecomastia and breast pain-in patients with prostate cancer.
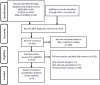
Methods: A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted according to PRISMA-P guidelines. A comprehensive search was performed in PubMed, Scopus, and Web of Science for English-language studies without temporal restrictions. Studies were included if they involved prostate cancer patients treated with bicalutamide receiving preventive interventions (tamoxifen, anastrozole, or radiotherapy) compared to bicalutamide alone (or bicalutamide plus placebo/sham). Data extraction focused on the incidence of gynecomastia and breast pain, and study quality was assessed using the Jadad scale. Risk ratios (RR) with 95% confidence intervals (CI) were calculated using fixed- or random-effects models, and heterogeneity was evaluated with the I² statistic. Publication bias was explored via funnel plots and the trim-and-fill method.
Results: Nine RCTs met the inclusion criteria. Tamoxifen significantly reduced the risk of breast events by 82% (RR: 0.18, 95% CI: 0.08-0.38 for gynecomastia and RR: 0.18, 95% CI: 0.07-0.43 for breast pain). Radiotherapy reduced gynecomastia risk by 52% (RR: 0.48, 95% CI: 0.38-0.59) and breast pain by 34% (RR: 0.66, 95% CI: 0.48-0.90). Anastrozole did not show significant benefit.
Conclusion: Tamoxifen appears to be the most effective strategy for preventing bicalutamide-induced breast events, with radiotherapy serving as a viable alternative, and anastrozole offering no benefit. Further large-scale, high-quality studies are needed to confirm these findings and refine preventive treatment recommendations.
Keywords: Aromatase; Bicalutamide; Gynecomastia; Radiotherapy; Tamoxifen; Testosterone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare they have no financial interests. Marco Giorgio Baroni and Arcangelo Barbonetti are members of the Editorial Board of the J Endocrinol Invest.
Figures




Similar articles
-
Tamoxifen for the management of breast events induced by non-steroidal antiandrogens in patients with prostate cancer: a systematic review.BMC Med. 2012 Aug 28;10:96. doi: 10.1186/1741-7015-10-96. BMC Med. 2012. PMID: 22925442 Free PMC article.
-
[Management strategies for gynecomastia in patients with prostate cancer treated with androgen receptor pathway inhibitors].Cancer Radiother. 2025 Jul;29(4):104665. doi: 10.1016/j.canrad.2025.104665. Epub 2025 Jun 24. Cancer Radiother. 2025. PMID: 40561872 Review. French.
-
Risk-reducing medications for primary breast cancer: a network meta-analysis.Cochrane Database Syst Rev. 2019 Apr 29;4(4):CD012191. doi: 10.1002/14651858.CD012191.pub2. Cochrane Database Syst Rev. 2019. PMID: 31032883 Free PMC article.
-
Gynecomastia in Patients with Prostate Cancer: A Systematic Review.PLoS One. 2015 Aug 26;10(8):e0136094. doi: 10.1371/journal.pone.0136094. eCollection 2015. PLoS One. 2015. PMID: 26308532 Free PMC article.
-
Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer.Cochrane Database Syst Rev. 2014 Jun 30;2014(6):CD009266. doi: 10.1002/14651858.CD009266.pub2. Cochrane Database Syst Rev. 2014. PMID: 24979481 Free PMC article.
References
-
- Perdonà S, Autorino R, De Placido S, D’Armiento M, Gallo A, Damiano R, Pingitore D, Gallo L, De Sio M, Bianco AR, Di Lorenzo G (2005) Efficacy of tamoxifen and radiotherapy for prevention and treatment of gynaecomastia and breast pain caused by bicalutamide in prostate cancer: a randomised controlled trial. Lancet Oncol.;6(5):295–300. 10.1016/S1470-2045(05)70103-0. PMID: 15863377 - PubMed
-
- Hamdy FC, Donovan JL, Lane JA, Metcalfe C, Davis M, Turner EL, Martin RM, Young GJ, Walsh EI, Bryant RJ, Bollina P, Doble A, Doherty A, Gillatt D, Gnanapragasam V, Hughes O, Kockelbergh R, Kynaston H, Paul A, Paez E, Powell P, Rosario DJ, Rowe E, Mason M, Catto JWF, Peters TJ, Oxley J, Williams NJ, Staffurth J, Neal DE (2023) ProtecT Study Group. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med.;388(17):1547–1558. 10.1056/NEJMoa2214122. Epub 2023 Mar 11. PMID: 36912538 - PubMed
-
- Iversen P (2002) Antiandrogen monotherapy: indications and results. Urology.;60(3 Suppl 1):64–71. 10.1016/s0090-4295(02)01576-5. PMID: 12231053 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

