Integrative genomic pan-cancer analysis reveals the prognostic significance of DEFB1 in tumors
- PMID: 40244529
- PMCID: PMC12006651
- DOI: 10.1007/s12672-025-02340-6
Integrative genomic pan-cancer analysis reveals the prognostic significance of DEFB1 in tumors
Abstract
Background: Defensin beta 1 (DEFB1) is a key immune response gene, but its role in cancer remains unclear. This study aims to explore DEFB1 expression, genetic alterations, immune infiltration, and prognostic significance across various cancer types.
Methods: We analyzed DEFB1 expression and its association with cancer prognosis using data from public platforms, including The Cancer Genome Atlas (TCGA), Genotype-Tissue Expression (GTEx), and Human Protein Atlas (HPA). Additionally, we examined DEFB1 genetic alterations, immune cell infiltration, and its molecular partners using various bioinformatics tools.
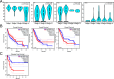
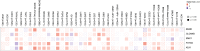
Results: DEFB1 expression was highest in salivary glands, kidneys, and pancreas. In cancers, DEFB1 was upregulated in cholangiocarcinoma, kidney chromophobe, and melanoma, but downregulated in breast, colon, and rectal cancers. High DEFB1 expression was linked to poorer overall survival in lung adenocarcinoma and pancreatic adenocarcinoma, but better survival in head and neck squamous cell carcinoma. Genetic analysis revealed alterations in liver and gastric cancers. Immune infiltration analysis showed a correlation between DEFB1 and cancer-associated fibroblasts in liver cancer, while neutrophil infiltration was linked to bladder carcinoma, diffuse large B-cell lymphoma, and lung squamous cell carcinoma. Key genes associated with DEFB1 included KLK1, BSND, and CLCNKB.
Discussion: This study highlights DEFB1's potential as a prognostic biomarker and its influence on the tumor immune microenvironment across different cancers. These findings suggest DEFB1 could be a target for future cancer therapies, although further studies are needed to validate these results.
Keywords: Bioinformatics; DEFB1; Immune infiltration; Malignancy; Prognosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the Ethics Committee of Tianjin Medical University Cancer Institute & Hospital (Approval No. EK20240003). All the experiments in this study were conducted in compliance with the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study. Competing interests: The authors declare no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
