Based on disulfidptosis, unveiling the prognostic and immunological signatures of Asian hepatocellular carcinoma and identifying the potential therapeutic target ZNF337-AS1
- PMID: 40244531
- PMCID: PMC12006654
- DOI: 10.1007/s12672-025-02325-5
Based on disulfidptosis, unveiling the prognostic and immunological signatures of Asian hepatocellular carcinoma and identifying the potential therapeutic target ZNF337-AS1
Abstract
Background: Disulfidptosis is a newly discovered programmed cell death pathway that may be connected to tumorigenesis and development, showing promise as a novel treatment strategy for cancer. This study aims to construct a prognostic model of disulfidptosis-related Long non-coding RNAs (DRLRs) within the Asian HCC population and to investigate the impact of DRLRs on HCC.
Methods: Utilising a combination of univariate Cox, Lasso-Cox, and multivariate Cox analyses, five pivotal DRLRs (AC099850.3, ZNF337-AS1, LINC01138, AL031985.3, AC131009.1) were identified, forming a robust prognostic signature. Subsequent validations included Receiver Operating Characteristic (ROC) and Concordance Index analyses, alongside Principal Component Analysis. Comprehensive bioinformatics analysis was performed on the hub DRLRs, followed by experimental validation using quantitative real-time polymerase chain reaction and cellular functional assays.
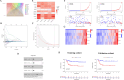
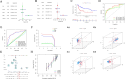
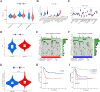
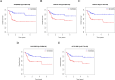
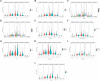
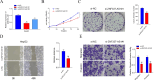
Results: The risk score independently predicted prognosis, outperforming traditional clinical-pathological factors across varying ages, tumour stages, and pathological classifications in the cohort. A nomogram integrating these variables demonstrated capability in forecasting survival. Multivariate analysis confirmed that the risk score and AJCC TNM staging are independent prognostic factors for predicting overall survival (OS) in Asian HCC patients (both P < 0.001). The prognostic model's ROC area under the ROC values for 1-, 3-, and 5-year predictions were 0.837, 0.794, and 0.783, respectively, indicating its strong diagnostic and prognostic value. Pathway and immune landscape analyses elucidated the biological underpinnings and immune modulations associated with the high-risk group. Immune landscape analysis indicated that both immunescore (P < 0.001) and estimatescore (P < 0.05) were significantly decreased in the high-risk group, with both specific and non-specific immune responses being significantly suppressed, while the tumour immune dysfunction and exclusion score was notably increased (P < 0.001). Tumour mutational burden (TMB) analysis revealed a significantly higher TMB in the high-risk group (P = 0.033) and shorter OS for HCC patients in the high TMB subgroup (P = 0.002). Notably, Potential chemotherapeutic agents (PFI3, 5-Fluorouracil, BPD-00008900, GDC0810, and AZ6102) were identified for high-risk group. Experimental validations through quantitative PCR and in vitro assays confirmed the deregulation of these DRLRs in HCC, with functional studies highlighting the potential of ZNF337-AS1 silencing in curtailing tumour invasiveness.
Conclusion: Our investigations validate a DRLR-based risk scoring model as an effective prognostic tool for Asian HCC. This model not only enhances understanding of disulfidptosis's role in HCC but also facilitates personalised treatment strategies, potentially improving patient outcomes.
Keywords: Bioinformatics; Disulfidptosis; Hepatocellular carcinoma; Long noncoding RNA; ZNF337-AS1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in line with the principles of the Declaration of Helsinki. The Ethics Committee of the jurong hospital affiliated to jiangsu university (zhenjiang, China) approved the present study (approval no. JRSRMYY-2023–042) of tumor research. Each patient provided written informed consent for participation. Animal ethics: Not applicable. Consent for publication: Not applicable. Conflicts of interest: The authors declare no competing interests.
Figures



Similar articles
-
Construction of a prognostic model for disulfidptosis-related long noncoding RNAs in R0 resected hepatocellular carcinoma and analysis of their impact on malignant behavior.BMC Cancer. 2024 Aug 29;24(1):1068. doi: 10.1186/s12885-024-12816-3. BMC Cancer. 2024. PMID: 39210306 Free PMC article.
-
Systematic Analysis of Disulfidptosis-Related lncRNAs in Hepatocellular Carcinoma with Vascular Invasion Revealed That AC131009.1 Can Promote HCC Invasion and Metastasis through Epithelial-Mesenchymal Transition.ACS Omega. 2024 Dec 3;9(50):49986-49999. doi: 10.1021/acsomega.4c09411. eCollection 2024 Dec 17. ACS Omega. 2024. PMID: 39713637 Free PMC article.
-
A Cuproptosis-Related LncRNA Risk Model for Predicting Prognosis and Immunotherapeutic Efficacy in Patients with Hepatocellular Carcinoma.Biochem Genet. 2024 Jun;62(3):2332-2351. doi: 10.1007/s10528-023-10539-x. Epub 2023 Oct 29. Biochem Genet. 2024. PMID: 37898914
-
Development of a novel disulfidptosis-related lncRNA signature for prognostic and immune response prediction in clear cell renal cell carcinoma.Sci Rep. 2024 Jan 5;14(1):624. doi: 10.1038/s41598-024-51197-2. Sci Rep. 2024. PMID: 38182642 Free PMC article.
-
Integrating bioinformatics and experimental validation to unveil disulfidptosis-related lncRNAs as prognostic biomarker and therapeutic target in hepatocellular carcinoma.Cancer Cell Int. 2024 Jan 13;24(1):30. doi: 10.1186/s12935-023-03208-x. Cancer Cell Int. 2024. PMID: 38218909 Free PMC article.
Cited by
-
A non-invasive nomogram for the prediction of poor prognosis of hepatocellular carcinoma based on the novel marker Interleukin-41.BMC Cancer. 2025 May 26;25(1):941. doi: 10.1186/s12885-025-14344-0. BMC Cancer. 2025. PMID: 40419967 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. - PubMed
-
- Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020;470:8–17. 10.1016/j.canlet.2019.12.002. - PubMed
LinkOut - more resources
Full Text Sources
