Materials Advances in Devices for Heart Disease Interventions
- PMID: 40244561
- PMCID: PMC12243731
- DOI: 10.1002/adma.202420114
Materials Advances in Devices for Heart Disease Interventions
Abstract
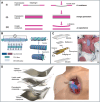
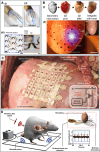
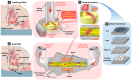
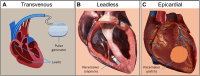
Heart disease encompasses a range of conditions that affect the heart, including coronary artery disease, arrhythmias, congenital heart defects, heart valve disease, and conditions that affect the heart muscle. Intervention strategies can be categorized according to when they are administered and include: 1) Monitoring cardiac function using sensor technology to inform diagnosis and treatment, 2) Managing symptoms by restoring cardiac output, electrophysiology, and hemodynamics, and often serving as bridge-to-recovery or bridge-to-transplantation strategies, and 3) Repairing damaged tissue, including myocardium and heart valves, when management strategies are insufficient. Each intervention approach and technology require specific material properties to function optimally, relying on materials that support their action and interface with the body, with new technologies increasingly depending on advances in materials science and engineering. This review explores material properties and requirements driving innovation in advanced intervention strategies for heart disease and highlights key examples of recent progress in the field driven by advances in materials research.
Keywords: biomaterials; cardiovascular disease; devices; heart disease; tissue engineering.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Martin S. S., Aday A. W., Almarzooq Z. I., Anderson C. A. M., Arora P., Avery C. L., Baker‐Smith C. M., Barone Gibbs B., Beaton A. Z., Boehme A. K., Commodore‐Mensah Y., Currie M. E., Elkind M. S. V., Evenson K. R., Generoso G., Heard D. G., Hiremath S., Johansen M. C., Kalani R., Kazi D. S., Ko D., Liu J., Magnani J. W., Michos E. D., Mussolino M. E., Navaneethan S. D., Parikh N. I., Perman S. M., Poudel R., Rezk‐Hanna M., et al., Circulation 2024, 149, 347.
-
- Townsend N., Kazakiewicz D., Lucy Wright F., Timmis A., Huculeci R., Torbica A., Gale C. P., Achenbach S., Weidinger F., Vardas P., Nat. Rev. Cardiol. 2022, 19, 133. - PubMed
-
- Mirzajani H., Kraft M., ACS Sens. 2024, 9, 4328. - PubMed
-
- Rothman S. A., Laughlin J. C., Seltzer J., Walia J. S., Baman R. I., Siouffi S. Y., Sangrigoli R. M., Kowey P. R., J. Cardiovasc. Electrophysiol. 2007, 18, 241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

