Verapamil and its metabolite norverapamil inhibit the Mycobacterium tuberculosis MmpS5L5 efflux pump to increase bedaquiline activity
- PMID: 40244664
- PMCID: PMC12036985
- DOI: 10.1073/pnas.2426827122
Verapamil and its metabolite norverapamil inhibit the Mycobacterium tuberculosis MmpS5L5 efflux pump to increase bedaquiline activity
Abstract
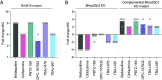
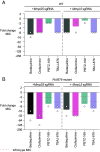
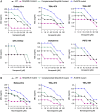
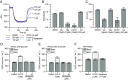
Bedaquiline is the cornerstone of a new regimen for the treatment of drug-resistant tuberculosis. However, its clinical use is threatened by the emergence of bedaquiline-resistant strains of Mycobacterium tuberculosis. Bedaquiline targets mycobacterial ATP synthase but the predominant route to clinical bedaquiline resistance is via upregulation of the MmpS5L5 efflux pump due to mutations that inactivate the transcriptional repressor Rv0678. Here, we show that the MmpS5L5 efflux pump reduces susceptibility to bedaquiline as well as its new, more potent derivative TBAJ-876 and other antimicrobial substrates, including clofazimine and the DprE1 inhibitors PBTZ-169 and OPC-167832. Furthermore, the increased resistance of Rv0678 mutants stems entirely from increased MmpS5L5 expression. These results highlight the potential of a pharmacological MmpS5L5 inhibitor to increase drug efficacy. Verapamil, primarily used as a calcium channel inhibitor, is known to inhibit diverse efflux pumps and to potentiate bedaquiline and clofazimine activity in M. tuberculosis. Here, we show that verapamil potentiates the activity of multiple diverse MmpS5L5 substrates. Using biochemical approaches, we demonstrate that verapamil does not exert this effect by acting as a disruptor of the protonmotive force used to power MmpS5L5, as previously proposed, suggesting that verapamil inhibits the function of the MmpS5L5 pump. Finally, norverapamil, the major verapamil metabolite, which has greatly reduced calcium channel activity, has equal potency in reducing resistance to MmpS5L5 substrates. Our findings highlight verapamil's potential for enhancing bedaquiline TB treatment, for preventing acquired resistance to bedaquiline and other MmpS5L5 substrates, while also providing the impetus to identify additional MmpS5L5 inhibitors.
Keywords: MmpS5L5 drug efflux pump; Mycobacterium tuberculosis; TBAJ-587 and TBAJ-876; bedaquiline; verapamil and norverapamil.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
-
- WHO. Global tuberculosis report 2022 (2022).
-
- WHO, WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment—Drug-Resistant Tuberculosis Treatment, 2022 Update (World Health Organization, 2022). - PubMed
-
- Nyang’wa B. T., et al. , A 24-week, all-oral regimen for rifampin-resistant tuberculosis. N. Engl. J. Med. 387, 2331–2343 (2022). - PubMed
-
- Nyang’wa B. T., et al. , Short oral regimens for pulmonary rifampicin-resistant tuberculosis (TB-PRACTECAL): An open-label, randomised, controlled, phase 2B–3, multi-arm, multicentre, non-inferiority trial. Lancet Respir. Med. 12, 117–128 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

