Cleavage cascade of the sigma regulator FecR orchestrates TonB-dependent signal transduction
- PMID: 40244679
- PMCID: PMC12036975
- DOI: 10.1073/pnas.2500366122
Cleavage cascade of the sigma regulator FecR orchestrates TonB-dependent signal transduction
Abstract
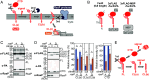
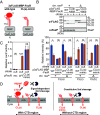
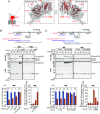
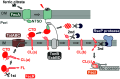
TonB-dependent signal transduction is a versatile mechanism observed in gram-negative bacteria that integrates energy-dependent substrate transport with signal relay. In Escherichia coli, the TonB-ExbBD motor complex energizes the TonB-dependent outer membrane transporter FecA, facilitating ferric citrate import. FecA also acts as a sensor, transmitting signals to the cytoplasmic membrane protein FecR, which eventually activates the cytoplasmic sigma factor FecI, driving transcription of the fec operon. Building on our previous finding that FecR undergoes functional maturation through a three-step cleavage process [T. Yokoyama et al., J. Biol. Chem. 296, 100673 (2021)], we here describe the complete mechanism of FecR-mediated ferric citrate signaling involving FecA and TonB. The cleavage cascade begins with FecR autoproteolysis prior to membrane integration. The soluble C-terminal domain (CTD) fragment of FecR is cotranslocated with the N-terminal domain (NTD) fragment through a twin-arginine translocation (Tat) system-mediated process. In the periplasm, the interaction between the CTD and NTD fragments prevents further cleavage. Binding of ferric citrate induces a conformational change in FecA, exposing its TonB box to the periplasmic space. This structural alteration is transmitted to the interacting FecR CTD via the motor function of TonB, resulting in the release of the CTD blockage from the NTD. Consequently, the successive cleavage of FecR's NTD is initiated, culminating in the ferric citrate signal-induced activation of fec gene expression. Our findings reveal that the regulation of FecR cleavage, controlled by the TonB-FecA axis, plays a central role in the bacterial response to ferric citrate signals.
Keywords: Fec system; Tat system; Ton system; iron transport; signal transduction.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Jacob F., Monod J., Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 3, 318–356 (1961). - PubMed
-
- Tetsch L., Jung K., The regulatory interplay between membrane-integrated sensors and transport proteins in bacteria. Mol. Microbiol. 73, 982–991 (2009). - PubMed
-
- Braun V., Braun M., Iron transport and signaling in Escherichia coli. FEBS Lett. 529, 78–85 (2002). - PubMed
-
- Hussein S., Hantke K., Braun V., Citrate-dependent iron transport system in Escherichia coli K-12. Eur. J. Biochem. 117, 431–437 (1981). - PubMed
MeSH terms
Substances
Grants and funding
- JP23KJ1581/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24H01370/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24K17817/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22K15061/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21H05155/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources

