Disruption of ataxia telangiectasia-mutated kinase enhances radiation therapy efficacy in spatially directed diffuse midline glioma models
- PMID: 40244686
- PMCID: PMC12165813
- DOI: 10.1172/JCI179395
Disruption of ataxia telangiectasia-mutated kinase enhances radiation therapy efficacy in spatially directed diffuse midline glioma models
Abstract
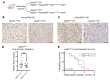
Diffuse midline gliomas (DMGs) are lethal brain tumors characterized by p53-inactivating mutations and oncohistone H3.3K27M mutations that rewire the cellular response to genotoxic stress. We used RCAS/tv-a retroviruses and Cre recombinase to inactivate p53 and induce native H3.3K27M mutations in a lineage- and spatially directed manner. We generated primary mouse tumors that recapitulated human DMG. Disrupting ataxia-telangiectasia mutated (ATM) kinase enhanced the efficacy of radiation therapy (RT) in murine and patient-derived DMG models and increased survival. Microscopy-based in situ sequencing was used to spatially resolve transcriptional profiles in more than 750,000 single cells with or without ATM disruption and RT, revealing altered immune-neoplastic and endothelial cell interactions after treatment. An allelic series of primary murine DMG models with different p53 mutations confirmed that transactivation-independent p53 activity was a key mediator of radiosensitivity after ATM disruption. We generated primary DMG mouse models and performed deep profiling that revealed mechanisms of response to ATM disruption and RT that can be utilized as a therapeutic strategy.
Keywords: Brain cancer; Drug therapy; Mouse models; Neuroscience; Oncology.
Figures
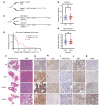

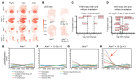

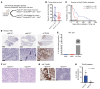
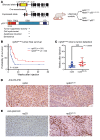
Update of
-
Ataxia-telangiectasia mutated ( Atm ) disruption sensitizes spatially-directed H3.3K27M/TP53 diffuse midline gliomas to radiation therapy.bioRxiv [Preprint]. 2023 Oct 20:2023.10.18.562892. doi: 10.1101/2023.10.18.562892. bioRxiv. 2023. Update in: J Clin Invest. 2025 Apr 17;135(12):e179395. doi: 10.1172/JCI179395. PMID: 37904990 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

