IL-7-mediated expansion of autologous lymphocytes increases CD8+ VLA-4 expression and accumulation in glioblastoma models
- PMID: 40244705
- PMCID: PMC12165802
- DOI: 10.1172/JCI181471
IL-7-mediated expansion of autologous lymphocytes increases CD8+ VLA-4 expression and accumulation in glioblastoma models
Abstract
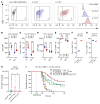
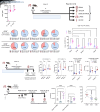
The efficacy of T cell-activating therapies against glioma is limited by an immunosuppressive tumor microenvironment and tumor-induced T cell sequestration. We investigated whether peripherally infused nonantigen specific autologous lymphocytes could accumulate in intracranial tumors. We observed that nonspecific autologous CD8+ ALT cells can indeed accumulate in this context, despite endogenous T cell sequestration in bone marrow. Rates of intratumoral accumulation were markedly increased when expanding lymphocytes with IL-7 compared with IL-2. Pretreatment with IL-7 ALT also enhanced the efficacy of multiple tumor-specific and nontumor-specific T cell-dependent immunotherapies against orthotopic murine and human xenograft gliomas. Mechanistically, we detected increased VLA-4 on mouse and human CD8+ T cells following IL-7 expansion, with increased transcription of genes associated with migratory integrin expression (CD9). We also observed that IL-7 increases S1PR1 transcription in human CD8+ T cells, which we have shown to be protective against tumor-induced T cell sequestration. These observations demonstrate that expansion with IL-7 enhances the capacity of ALT to accumulate within intracranial tumors and that pretreatment with IL-7 ALT can boost the efficacy of subsequent T cell-activating therapies against glioma. Our findings will inform the development of future clinical trials where ALT pretreatment can be combined with T cell-activating therapies.
Keywords: Cell migration/adhesion; Immunotherapy; Neuroscience; Oncology; T cells.
Figures





Update of
-
IL-7-mediated expansion of autologous lymphocytes increases CD8+ VLA-4 expression and accumulation in glioblastoma models.bioRxiv [Preprint]. 2025 Apr 14:2024.04.01.587634. doi: 10.1101/2024.04.01.587634. bioRxiv. 2025. Update in: J Clin Invest. 2025 Apr 17;135(12):e181471. doi: 10.1172/JCI181471. PMID: 41031006 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

