Straining Flow Effects on Sperm Flagellar Energetics in Microfluidic Cross-Slot Traps
- PMID: 40244872
- PMCID: PMC12105419
- DOI: 10.1002/smll.202500813
Straining Flow Effects on Sperm Flagellar Energetics in Microfluidic Cross-Slot Traps
Abstract
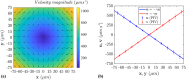
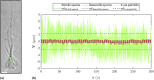
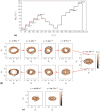
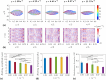
Sperm need to effectively navigate the intricate pathways of the female reproductive tract, which are filled with various complex fluid flows. Despite numerous population-based studies, the effects of flow on the flagellar beating pattern of individual sperm remain poorly understood. In this study, a microfluidic cross-slot trap is employed to immobilize individual motile sperm for an extended period without physical tethering, thereby reducing potential cell damage and movement restriction compared to the conventional head-tethering method. The impact of pure straining flow on trapped single sperm is investigated. The experimental results demonstrate that at strain rates of 11.33 s-1 and higher, the periodic and repetitive beating pattern of the sperm flagellum changes to irregular movement. Furthermore, an increase in strain rate from 1.89 to 11.33 s-1 leads to a 35.4% reduction in beating amplitude and a 41.2% decrease in hydrodynamic power dissipation. These findings underscore the capability of the microfluidic cross-slot platform to trap sperm with high stability, contributing to a better understanding of sperm behavior in response to fluid flows.
Keywords: microfluidic cross‐slot trap; single sperm trapping; sperm flagellar beating behavior; straining flow.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Microfluidic sperm trap array for single-cell flagellar analysis with unrestricted 2D flagellar movement.Lab Chip. 2024 Oct 9;24(20):4827-4842. doi: 10.1039/d4lc00515e. Lab Chip. 2024. PMID: 39291409
-
Are there intracellular Ca2+ oscillations correlated with flagellar beating in human sperm? A three vs. two-dimensional analysis.Mol Hum Reprod. 2017 Sep 1;23(9):583-593. doi: 10.1093/molehr/gax039. Mol Hum Reprod. 2017. PMID: 28911211
-
Regulatory mechanisms of sperm flagellar motility by metachronal and synchronous sliding of doublet microtubules.Mol Hum Reprod. 2017 Dec 1;23(12):817-826. doi: 10.1093/molehr/gax055. Mol Hum Reprod. 2017. PMID: 29040653
-
Microscopic analysis of sperm movement: links to mechanisms and protein components.Microscopy (Oxf). 2018 Jun 1;67(3):144-155. doi: 10.1093/jmicro/dfy021. Microscopy (Oxf). 2018. PMID: 29741637 Review.
-
Digital image analysis of flagellar beating and microtubule sliding of activated and hyperactivated sperm flagella.Soc Reprod Fertil Suppl. 2007;65:327-30. Soc Reprod Fertil Suppl. 2007. PMID: 17644972 Review.
References
-
- Tabalvandani M. B., Javadizadeh S., Badieirostami M., Lab Chip 2024, 24, 1636. - PubMed
-
- Nosrati R., Lab Chip 2022, 22, 1680. - PubMed
-
- Nath B., Caprini L., Maggi C., Zizzari A., Arima V., Viola I., Di Leonardo R., Puglisi A., Lab Chip 2023, 23, 773. - PubMed
-
- Simchi M., Riordon J., You J. B., Wang Y., Xiao S., Lagunov A., Hannam T., Jarvi K., Nosrati R., Sinton D., Lab Chip 2021, 21, 2464. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

